Comparative efficacy and safety of first-line EGFR-TKIs for advanced non-small cell lung cancer:a network meta-analysis
-
摘要:
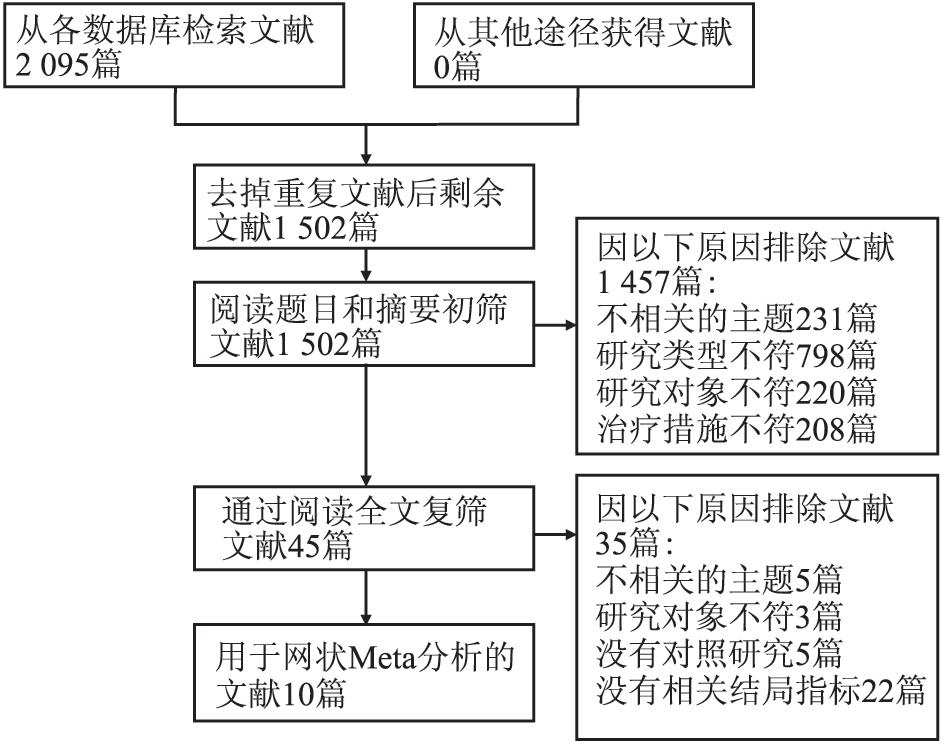
目的 比较吉非替尼、厄洛替尼和阿法替尼一线治疗晚期非小细胞肺癌(non-small cell lung cancer, NSCLC)的有效性和安全性。 方法 系统检索2008年12月-2018年12月收录在PubMed、EMBASE和The Cochrane Library中的相关文献进行贝叶斯网状meta分析。 结果 共纳入10篇文献, 包含2 275名患者。就有效性而言, 累积排序概率图下面积(surface under the cumulative ranking, SUCRA)显示厄洛替尼在无进展生存期(progression-free survival, PFS)方面最佳(0.88), 阿法替尼在客观反应率(objective response rate, ORR)(0.82)和疾病控制率(disease control rate, DCR)(0.86)方面最佳, 吉非替尼在PFS(0.45), ORR(0.42)和DCR(0.45)方面均最差。就安全性而言, 仅厄洛替尼与含铂的双重化疗在3~4级不良反应率(OR=0.29, 95% CI:0.08~0.98)和停药率(OR=0.14, 95% CI:0.01~0.86)方面的差异有统计学意义。排序结果也支持厄洛替尼的安全性最好。SUCRA结果提示吉非替尼(0.31)发生3~4级不良反应的可能性比阿法替尼(0.57)小, 其(0.44)发生停药的可能性与阿法替尼(0.41)相似。 结论 厄洛替尼可能是三者中一线治疗晚期NSCLC的首选药物。 Abstract:Objective To compare the efficacy and safety of gefitinib, erlotinib, and afatinib in the first-line treatment of advanced non-small cell lung cancer(NSCLC). Methods PubMed, EMBASE, and The Cochrane Library were searched to identify the relevant literatures published from December 2008 to December 2018. Bayesian network meta-analysis was carried out to rank the three treatments. Results A total of ten eligible studies involving 2275 patients were enrolled. In terms of efficacy, the surface under the cumulative ranking(SUCRA) indicated that erlotinib performed best in progression-free survival(PFS)(0.88), afatinib performed best in objective response rate(ORR)(0.82) and disease control rate(DCR)(0.86), gefitinib performed worst in PFS(0.45), ORR(0.42), and DCR(0.45). For safety, the differences of grade 3 or 4 adverse events rate(OR=0.29, 95%CI:0.08-0.98) and discontinuation rate(OR=0.14, 95%CI:0.01-0.8) between erlotinib and the platinum-based doublet chemotherapy were statistically significant. The ranking results also supported that erlotinib was the safest. SUCRA results suggested that gefitinib(0.31) had a lower grade 3 or 4 adverse events rate than afatinib(0.57), and the possibility of discontinuation in gefitinib(0.44) was similar to that of afatinib(0.41). Conclusion Erlotinib might be the preferred first-line treatment for advanced NSCLC after weighing and balancing the benefits and risks. -
Key words:
- Non-small cell lung cancer /
- Gefitinib /
- Erlotinib /
- Afatinib /
- First-line treatment /
- Network meta-analysis
-
表 1 纳入研究的基本特征
Table 1. Characteristics of the included trials
纳入研究 种族 治疗措施 例数 中位年龄(岁) 女性占比(%) 非吸烟者占比(%) 腺癌占比(%) IPASS(2009, Ⅲ) 亚洲 吉非替尼 132 - 81.8 93.9 - 卡铂+紫杉醇 129 - 79.8 94.6 - NEJ002(2010, Ⅲ) 亚洲 吉非替尼 114 63.9 63.2 65.8 90.4 卡铂+紫杉醇 114 62.6 64 57.9 96.5 WJTOG3405(2010, Ⅲ) 亚洲 吉非替尼 86 64 68.6 70.9 96.5 顺铂+多烯紫杉醇 86 64 69.8 66.3 97.7 OPTIMAL(2011, Ⅲ) 亚洲 厄洛替尼 82 57 58.5 72 87.8 吉西他滨+卡铂 72 59 59.7 69.4 86.1 First-SIGNAL(2012, Ⅲ) 亚洲 吉非替尼 26 - - 100 100 吉西他滨+顺铂 16 - - 100 100 EURTAC(2012, Ⅲ) 欧洲 厄洛替尼 86 63.4 67.4 66.3 95.3 顺铂+多烯紫杉醇/吉西他滨 87 64.2 78.2 72.4 89.7 LUX-Lung 3(2013, Ⅲ) 全球 阿法替尼 230 61.5 63.9 67.4 100 顺铂+培美曲塞 115 61 67 70.4 100 LUX-Lung 6(2014, Ⅲ) 亚洲 阿法替尼 242 58 64 74.8 100 吉西他滨+顺铂 122 58 68 81.1 100 ENSURE(2015, Ⅲ) 亚洲 厄洛替尼 110 57.5 61.8 71.8 94.5 顺铂+吉西他滨 107 56 60.7 69.2 94.4 LUX-Lung 7(2016, ⅡB) 全球 阿法替尼 160 63 56.9 66.3 99.4 吉非替尼 159 63 66.7 66.7 99.4 注:“-”表示数据缺失。 表 2 点分法分析结果
Table 2. The results of node-splitting analysis
直接比较结果 间接比较结果 合并结果 P值 PFS 2 vs.1 -0.84(-1.40, -0.27) -0.59(-1.90, 0.75) -0.80(-1.30, -0.33) 0.664 4 vs.1 -0.90(-1.70, -0.13) -1.20(-2.40, 0.08) -0.97(-1.60, -0.39) 0.664 4 vs.2 -0.31(-1.40, 0.76) -0.06(-1.00, 0.87) -0.17(-0.82, 0.46) 0.673 ORR 2 vs.1 4.40(2.30, 9.20) 3.00(0.66, 14.00) 4.00(2.40, 7.30) 0.561 4 vs.1 5.60(2.30, 14.00) 8.10(2.10, 35.00) 6.10(3.20, 12.00) 0.563 4 vs.2 1.80(0.53, 6.50) 1.30(0.38, 3.70) 1.50(0.72, 3.00) 0.572 DCR 2 vs.1 2.20(1.10~4.90) 1.90(0.44~8.40) 2.10(1.20~3.90) 0.829 4 vs.1 2.90(1.30, 6.60) 3.40(0.84, 15.00) 3.00(1.60, 5.60) 0.830 4 vs.2 1.50(0.45, 5.10) 1.30(0.41, 4.00) 1.40(0.68, 2.80) 0.829 3~4级不良反应率 2 vs.1 0.27(0.02, 3.00) 0.52(0.03, 9.90) 0.36(0.07, 1.80) 0.658 4 vs.1 0.62(0.11, 3.50) 0.33(0.01, 10.00) 0.55(0.15, 2.10) 0.659 4 vs.2 1.20(0.11, 14.00) 2.30(0.11, 44.00) 1.50(0.30, 8.10) 0.671 注:对PFS, 表中所示为合并的HR的自然对数值及其95%CI; 对其他结局指标, 表中所示为合并的OR值及其95%CI。1=含铂的双重化疗, 2=吉非替尼, 4=阿法替尼; HR=风险比, OR=优势比, CI=置信区间。 表 3 各结局指标中不同治疗措施的相对治疗效果
Table 3. The relative treatment effects of different treatments for each outcome measure[4]
A PFS 阿法替尼 1.30(0.56~2.90) 厄洛替尼 0.84(0.44~1.60) 0.66(0.31~1.40) 吉非替尼 0.38(0.21~0.67) 0.30(0.16~0.53) 0.45(0.28~0.72) 含铂的双重化疗 B ORR 阿法替尼 1.00(0.40~2.60) 厄洛替尼 1.50(0.72~3.00) 1.50(0.60~3.40) 吉非替尼 6.10(3.20~12.00) 5.90(3.10~12.00) 4.00(2.40~7.30) 含铂的双重化疗 C DCR 阿法替尼 1.10(0.62~2.10) 厄洛替尼 1.40(0.87~2.30) 1.20(0.66~2.30) 吉非替尼 3.00(2.00~4.40) 2.60(1.60~4.20) 2.10(1.40~3.20) 含铂的双重化疗 D 3~4级不良反应率 阿法替尼 1.90(0.31~12.00) 厄洛替尼 1.50(0.30~8.00) 0.81(0.10~6.30) 吉非替尼 0.55(0.14~2.10) 0.29(0.08~0.98) 0.36(0.07~1.80) 含铂的双重化疗 E停药率 阿法替尼 1.70(0.11~44.00) 厄洛替尼 0.99(0.05~22.00) 0.58(0.01~34.00) 吉非替尼 0.24(0.03~2.10) 0.14(0.01~0.86) 0.24(0.01~11.00) 含铂的双重化疗 注:结果以合并的HR/OR及其95% CI表示(列治疗措施vs.行治疗措施)。对于A, HR < 1支持列治疗措施。对于B和C, OR > 1支持列治疗措施。对于D和E, OR < 1支持列治疗措施。HR=风险比; OR=优势比。 表 4 各结局指标中不同治疗措施的排序概率和SUCRA值
Table 4. The rank probabilities and SUCRAs of different treatments for each outcome measure
治疗措施 排序第一 排序第二 排序第三 排序第四 SUCRA值 A PFS 含铂的双重化疗 0.00 0.00 0.01 0.99 0.00 吉非替尼 0.05 0.26 0.68 0.00 0.45 厄洛替尼 0.72 0.20 0.08 0.00 0.88 阿法替尼 0.23 0.54 0.23 0.00 0.66 B ORR 含铂的双重化疗 0.00 0.00 0.00 1.00 0.00 吉非替尼 0.04 0.19 0.77 0.00 0.42 厄洛替尼 0.44 0.41 0.15 0.00 0.76 阿法替尼 0.53 0.40 0.08 0.00 0.82 C DCR 含铂的双重化疗 0.00 0.00 0.00 1.00 0.00 吉非替尼 0.05 0.25 0.71 0.00 0.45 厄洛替尼 0.32 0.44 0.24 0.00 0.69 阿法替尼 0.64 0.32 0.05 0.00 0.86 D 3~4级不良反应率 含铂的双重化疗 0.80 0.16 0.04 0.00 0.92 吉非替尼 0.06 0.17 0.42 0.35 0.31 厄洛替尼 0.02 0.14 0.28 0.56 0.20 阿法替尼 0.12 0.54 0.26 0.08 0.57 E停药率 含铂的双重化疗 0.77 0.18 0.05 0.00 0.91 吉非替尼 0.18 0.27 0.25 0.31 0.44 厄洛替尼 0.01 0.22 0.24 0.53 0.24 阿法替尼 0.04 0.33 0.46 0.17 0.41 注:SUCRA=累积排序概率图下面积。 -
[1] Zhang Y, Zhang Z, Huang X, et al. Therapeutic efficacy comparison of 5 major EGFR-TKIs in advanced EGFR-positive non-small-cell lung cancer: a network meta-analysis based on head-to-head trials[J]. Clin Lung Cancer, 2017, 18(5): e333-e340. DOI: 10.1016/j.cllc.2016.09.006. [2] Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer[J]. Mol Cancer, 2018, 17(1): 38. DOI: 10.1186/s12943-018-0777-1. [3] Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up[J]. Ann Oncol, 2019, 30(5): 863-870. DOI: 10.1093/annonc/mdy474. [4] Jansen JP, Fleurence R, Devine B, et al. Interpreting indirect treatment comparisons and network meta-analysis for health-care decision making: report of the ISPOR Task Force on Indirect Treatment Comparisons Good Research Practices: part 1[J]. Value Health, 2011, 14(4): 417-428. DOI: 10.1016/j.jval.2011.04.002. [5] Bhatnagar N, Lakshmi PV, Jeyashree K. Multiple treatment and indirect treatment comparisons: An overview of network meta-analysis[J]. Perspect Clin Res, 2014, 5(4): 154-158. DOI: 10.4103/2229-3485.140550. [6] Hong H, Carlin BP, Shamliyan TA, et al. Comparing Bayesian and frequentist approaches for multiple outcome mixed treatment comparisons[J]. Med Decis Making, 2013, 33(5): 702-714. DOI: 10.1177/0272989X13481110. [7] Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials[J]. BMJ, 2011, 343: d5928. DOI: 10.1136/bmj.d5928. [8] 易跃雄, 张蔚, 刘小媛, 等.网状Meta分析图形结果解读[J].中国循证医学杂志, 2015, 15(1): 103-109. DOI: 10.7507/1672-2531.20140263.Yi YX, Zhang W, Liu XY, et al. Result Interpretation of Network Meta-analysis[J]. Chin J Evid-Based Med, 2015, 15(1): 103-109. DOI: 10.7507/1672-2531.20140263. [9] Hoaglin DC, Hawkins N, Jansen JP, et al. Conducting indirect-treatment-comparison and network-meta-analysis studies: report of the ISPOR task force on indirect treatment comparisons good research practices: part 2[J]. Value Health, 2011, 14(4): 429-437. DOI: 10.1016/j.jval.2011.01.011. [10] Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial[J]. J Clin Epidemiol, 2011, 64(2): 163-171. DOI: 10.1016/j.jclinepi.2010.03.016. [11] van Valkenhoef G, Dias S, Ades AE, et al. Automated generation of node-splitting models for assessment of inconsistency in network meta-analysis[J]. Res Synth Methods, 2016, 7(1): 80-93. DOI: 10.1002/jrsm.1167. [12] Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma[J]. N Engl J Med, 2009, 361(10): 947-957. DOI: 10.1056/NEJMoa0810699. [13] Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR[J]. N Engl J Med, 2010, 362(25): 2380-2388. DOI: 10.1056/NEJMoa0909530. [14] Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor(WJTOG3405): an open label, randomised phase 3 trial[J]. Lancet Oncol, 2010, 11(2): 121-128. DOI: 10.1016/s1470-2045(09)70364-x. [15] Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer(OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study[J]. Lancet Oncol, 2011, 12(8): 735-742. DOI: 10.1016/s1470-2045(11)70184-x. [16] Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung[J]. J Clin Oncol, 2012, 30(10): 1122-1128. DOI: 10.1200/jco.2011.36.8456. [17] Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer(EURTAC): a multicentre, open-label, randomised phase 3 trial[J]. Lancet Oncol, 2012, 13(3): 239-246. DOI: 10.1016/s1470-2045(11)70393-x. [18] Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations[J]. J Clin Oncol, 2013, 31(27): 3327-3334. DOI: 10.1200/jco.2012.44.2806. [19] Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations(LUX-Lung 6): an open-label, randomised phase 3 trial[J]. Lancet Oncol, 2014, 15(2): 213-222. DOI: 10.1016/s1470-2045(13)70604-1. [20] Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study[J]. Ann Oncol, 2015, 26(9): 1883-1889. DOI: 10.1093/annonc/mdv270. [21] Park K, Tan EH, O'Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer(LUX-Lung 7): a phase 2B, open-label, randomised controlled trial[J]. Lancet Oncol, 2016, 17(5): 577-589. DOI: 10.1016/s1470-2045(16)30033-x. [22] Zhang Y, Sheng J, Yang Y, et al. Optimized selection of three major EGFR-TKIs in advanced EGFR-positive non-small cell lung cancer: a network meta-analysis[J]. Oncotarget, 2016, 7(15): 20093-20108. DOI: 10.18632/oncotarget.7713. [23] Riley RD, Dan J, Georgia S, et al.多重结局的多元Meta分析和多重治疗的网状Meta分析:原理、概念及实例[J].英国医学杂志中文版, 2018, 21(12): 725-735. DOI: 10.3760/cma.j.issn.1007-9742.2018.12.117.Riley RD, Dan J, Georgia S, et al. Meta-analysis of multiple outcomes and network meta-analysis of multiple treatments: principles, concepts, and examples[J]. The BMJ Chinese Edition, 2018, 21(12): 725-735. DOI: 10.3760/cma.j.issn.1007-9742.2018.12.117. [24] Yang Z, Hackshaw A, Feng Q, et al. Comparison of gefitinib, erlotinib and afatinib in non-small cell lung cancer: A meta-analysis[J]. Int J Cancer, 2017, 140(12): 2805-2819. DOI: 10.1002/ijc.30691. [25] Lin JZ, Ma SK, Wu SX, et al. A network meta-analysis of nonsmall-cell lung cancer patients with an activating EGFR mutation: Should osimertinib be the first-line treatment[J]. Medicine(Baltimore), 2018, 97(30): e11569. DOI: 10.1097/md.0000000000011569. [26] 田金徽, 李伦.网状Meta分析方法与实践[M].北京: 中国医药科技出版社, 2017.Tian JH, Li L. The method and practice of network meta-analysis[M]. Beijing: China Medical Science Press, 2017. [27] Kucharczuk CR, Ganetsky A, Vozniak JM. Drug-drug interactions, safety, and pharmacokinetics of EGFR tyrosine kinase inhibitors for the treatment of non-small cell lung cancer[J]. J Adv Pract Oncol, 2018, 9(2): 189-200. [28] Bronte G, Rolfo C, Giovannetti E, et al. Are erlotinib and gefitinib interchangeable, opposite or complementary for non-small cell lung cancer treatment?Biological, pharmacological and clinical aspects[J]. Crit Rev Oncol Hematol, 2014, 89(2): 300-313. DOI: 10.1016/j.critrevonc.2013.08.003. -





 下载:
下载: