Identification for biomarkers of skin lesions and lupus nephritis in patients with systemic lupus erythematosus
-
摘要:
目的 探索系统性红斑狼疮(systemic lupus erythematosus, SLE)患者皮肤损伤和狼疮性肾炎(lupus nephritis, LN)的蛋白质生物标志物,为SLE的诊断和控制提供依据。 方法 采用高通量蛋白质芯片技术检测SLE患者血清蛋白质生物标志物,针对皮肤损伤和LN这2种并发症对生物标志物进行单独和联合分析,寻找最佳诊断指标。 结果 单个指标转录因子5(transcription factor 5, SP5)、核糖体蛋白L7a(ribosomal protein L7a, RPL7A)以及联合指标[RPL7A+小核糖核蛋白多肽C(small nuclear ribonucleoprotein polypeptide C, SNRPC)]在皮肤损伤组及其对照组中具有最高的诊断价值。LN组与非LN组相比,SP5和[SP5+核糖体蛋白L35(ribosomal protein L35, RPL35)]的诊断价值最高。 结论 SP5可作为SLE患者皮肤损伤和LN诊断的蛋白质生物标志物。 Abstract:Objective To explore protein biomarkers of skin lesions and lupus nephritis (LN) in patients with systemic lupus erythematosus (SLE), so as to provide evidence for diagnosis and control of the SLE. Methods High-throughput protein chip technology was used to identify serum protein biomarkers for SLE, and the biomarkers were analyzed individually and jointly for the two complications of skin lesions and LN to find the best diagnostic indicators. Results The separate indicators transcription factor 5 (SP5), ribosomal protein L7a (RPL7A) and combined panel of (RPL7A +small nuclear ribonucleoprotein polypeptide C(SNRPC)) had the highest diagnostic value between skin lesions group and its control group. Compared with non-LN group, SP5 and (SP5+ribosomal protein L35(RPL35)) had the highest diagnostic value in LN group. Conclusion SP5 can be used as a protein biomarker for diagnosis of skin lesions and LN in SLE patients. -
Key words:
- Systemic lupus erythematosus /
- Skin lesions /
- Lupus nephritis /
- Biomarkers
-
表 1 不同组别的SLE患者人口统计学和实验室检测结果[n(%)]
Table 1. Demographics and laboratory test results of different groups of SLE patients [n(%)]
变量 总SLE患者(n=107) 皮损组(n=48) 非皮损组(n=59) t/χ2/U值 P值 LN组(n=49) 非LN组(n=58) t/χ2/U值 P值 人口学特征 年龄(x±s, 岁) 34.36±11.56 32.96±11.13 35.51±11.87 1.136 0.258 34.92±10.32 33.90±12.59 0.454 0.651 性别 1.593 0.207 0.013 0.909 男 17(15.89) 10(20.83) 7(11.86) 8(16.33) 9(15.52) 女 90(84.11) 38(79.17) 52(88.14) 41(83.67) 49(84.48) BMI(x±s, kg/m2) 21.77±3.36 21.64±3.39 21.87±3.36 0.350 0.727 22.65±3.44 20.25±4.96 2.863 0.005 病程[M(P25, P75), 年] 3.73(0.50, 8.50) 3.68(0.21, 8.79) 3.77(1.35, 8.37) 0.070 0.945 3.49(0.54, 7.48) 3.77(0.42, 9.66) 0.943 0.348 SLEDAI-2K评分(x±s, 分) 14.14±6.29 14.79±6.33 13.61±6.25 0.967 0.336 16.10±6.26 12.48±5.87 3.015 0.003 实验室检测指标 抗-dsDNA抗体a [M(P25, P75), IU/L] 291.00
(84.10, 685.73)215.44
(102.00, 585.00)420.50
(43.94, 800.00)1.135 0.259 511.00
(207.54, 689.00)154.00
(55.28, 706.60)1.257 0.212 抗-Sm抗体b 1.365 0.243 0.035 0.851 阳性 43(40.19) 21(43.75) 22(37.29) 19(38.78) 24(41.38) 阴性 64(59.81) 27(56.25) 37(62.71) 30(61.22) 34(58.62) 抗-SSA抗体c 0.329 0.566 0.329 0.566 阳性 61(57.01) 29(60.42) 32(54.24) 29(59.18) 32(55.17) 阴性 46(42.99) 19(39.58) 27(45.76) 20(40.82) 26(44.83) 抗-SSB抗体d 0.581 0.446 0.588 0.443 阳性 19(17.76) 10(20.83) 9(15.25) 7(14.29) 12(20.69) 阴性 88(82.24) 38(79.17) 50(84.75) 42(85.71) 46(79.31) 抗-RNP抗体e 0.106 0.745 0.005 0.946 阳性 54(50.47) 25(52.08) 29(49.15) 24(48.98) 30(51.72) 阴性 53(49.53) 23(47.92) 30(50.85) 25(51.02) 28(48.28) 抗-核糖体P抗体 1.207 0.272 0.805 0.370 阳性 43(40.19) 22(45.83) 21(35.59) 17(34.69) 26(44.83) 阴性 64(59.81) 26(54.17) 38(64.41) 32(65.31) 32(55.17) 血清C3(x±s, g/L) 0.55±0.24 0.52±0.24 0.57±0.24 1.109 0.271 0.48±0.25 0.61±0.22 2.699 0.008 血清C4[M(P25, P75), g/L] 0.09(0.05, 0.16) 0.09(0.04, 0.16) 0.10(0.05, 0.16) 1.651 0.102 0.05(0.03, 0.12) 0.12(0.07, 0.18) 2.304 0.024 ESR f(x±s, mm/h) 45.52±30.20 44.54±31.02 46.33±29.76 0.294 0.769 40.61±26.50 49.62±32.65 1.502 0.136 CRP g[M(P25, P75), g/L] 4.78(3.01, 11.52) 3.82(2.10, 10.93) 6.69(3.06, 14.98) 0.638 0.525 3.99(1.10, 9.18) 5.61(3.11, 22.16) 2.470 0.016 IgG(x±s, g/L) 13.16±6.70 13.93±7.17 12.52±6.29 1.042 0.300 11.45±6.51 14.47±6.61 2.269 0.025 IgM[M(P25, P75), g/L] 1.12(0.78, 1.46) 1.12(0.79, 1.48) 1.09(0.76, 1.46) 1.749 0.087 1.04(0.65, 1.45) 1.13(0.86, 1.47) 0.295 0.769 IgA(x±s, g/L) 2.79±1.44 2.72±1.34 2.84±1.53 0.438 0.662 2.40±0.81 3.07±1.72 2.293 0.024 注:a抗-dsDNA抗体:抗-double stranded DNA抗体,抗-双链DNA抗体;b抗-Sm抗体:抗-Smith抗体;c抗-SSA抗体:抗-Sjögren's syndrome-related antigen A抗体;d抗-SSB抗体:抗-Sjögren's syndrome-related antigen B抗体;e抗-RNP抗体:抗-ribonucleoprotein抗体;fESR:erythrocyte sedimentation rate,红细胞沉降率;gCRP:C-reactive protein,C反应蛋白。 表 2 皮损组与非皮损组之间指标的统计学差异
Table 2. Statistical differences in indicators between skin lesion group and non-lesion group
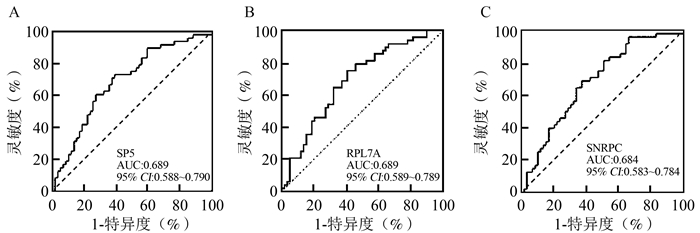
自身抗体 英文名称 中文名称 编号 AUC(95% CI)值 P值 SP5 transcription factor 5 转录因子5 JHU16770.P207C03 0.689(0.588~0.790) 0.001 RPL7A ribosomal protein L7a 核糖体蛋白L7a JHU03725.P039F07 0.689(0.589~0.789) 0.001 SNRPC small nuclear ribonucleoprotein polypeptide C 小核糖核蛋白多肽C JHU10916.P114F04 0.684(0.583~0.784) 0.001 MAK16 MAK16 homolog MAK16同系物 JHU15360.P161C08 0.679(0.579~0.780) 0.001 RPL35 ribosomal protein L35 核糖体蛋白L35 JHU09862.P103B12 0.675(0.573~0.777) 0.002 RPLP1 ribosomal protein lateral stalk subunit P1 核糖体蛋白侧茎亚基P1 JHU00457.P005H04 0.670(0.571~0.774) 0.003 RPL23A ribosomal protein L23a 核糖体蛋白L23a JHU04969.P052B07 0.667(0.566~0.769) 0.003 RPL14 ribosomal protein L14 核糖体蛋白L14 JHU03817.P040E04 0.665(0.563~0.766) 0.003 RPLP2 ribosomal protein lateral stalk subunit P2 核糖体蛋白侧茎亚基P2 JHU02847.P030F03 0.648(0.543~0.754) 0.009 RPLP0 ribosomal protein lateral stalk subunit P0 核糖体蛋白侧茎亚基P0 JHU16464.P173H05 0.641(0.536~0.746) 0.012 JUND AP-1 transcription factor subunit AP-1转录因子亚基 JHU16748.P207G04 0.640(0.534~0.746) 0.013 RELL1 RELT like 1 - JHU07369.P080C05 0.636(0.527~0.745) 0.016 LLPH long-term synaptic facilitation factor 长期突触促进因子 JHU07686.P081G04 0.633(0.527~0.739) 0.018 H1F0 H1.0 linker histone H1.0接头组蛋白 JHU05317.P056B04 0.629(0.522~0.735) 0.023 DBP D-box binding PAR bZIP transcription factor D-box结合PAR bZIP转录因子 JHU16638.P206A06 0.626(0.521~0.732) 0.025 TBX1 T-box transcription factor 1 T-box转录因子1 JHU16772.P207H12 0.620(0.511~0.729) 0.034 PIK3CG phosphatidylinositol-4, 5-bisphosphate 3-kinase catalytic subunit gamma 磷脂酰肌醇-4, 5-二磷酸3-激酶催化亚基γ JHU19507.P198B03 0.617(0.508~0.725) 0.039 FOXC2 forkhead box C2 叉头框C2 JHU19731.P182F10 0.613(0.505~0.721) 0.044 HIST1H1C H1.2 linker histone H1.2接头组蛋白 JHU02441.P026E04 0.612(0.505~0.720) 0.046 CDK19 cyclin dependent kinase 19 细胞周期蛋白依赖性激酶19 JHU09906.P191C06 0.611(0.502~0.720) 0.048 表 3 LN组与非LN组之间指标的统计学差异
Table 3. Statistical differences in indicators between LN group and non-LN group
自身抗体 编号 AUC(95% CI)值 P值 SP5 JHU16770.P207C03 0.676(0.574~0.778) 0.002 RPL7A JHU03725.P039F07 0.664(0.560~0.767) 0.004 RPL35 JHU09862.P103B12 0.663(0.559~0.766) 0.004 MAK16 JHU15360.P161C08 0.652(0.548~0.756) 0.007 RPL14 JHU03817.P040E04 0.647(0.542~0.753) 0.009 RPL23A JHU04969.P052B07 0.641(0.536~0.745) 0.012 LLPH JHU07686.P081G04 0.640(0.535~0.745) 0.013 SNRPC JHU10916.P114F04 0.639(0.533~0.745) 0.013 RPLP1 JHU00457.P005H04 0.638(0.530~0.745) 0.014 RPLP0 JHU16464.P173H05 0.634(0.526~0.742) 0.018 JUND JHU16748.P207G04 0.628(0.522~0.734) 0.023 PIK3CG JHU19507.P198B03 0.623(0.517~0.729) 0.029 RELL1 JHU07369.P080C05 0.620(0.513~0.727) 0.033 GABRA4 a JHU05993.P063H11 0.620(0.514~0.726) 0.032 RPLP2 JHU02847.P030F03 0.616(0.507~0.726) 0.038 TBX1 JHU16772.P207H12 0.613(0.505~0.720) 0.045 注:aGABRA4:gamma-aminobutyric acid type A receptor subunit alpha4,γ-氨基丁酸A型受体亚基alpha4。 表 4 具有较高诊断价值的单个指标结果
Table 4. Results in single indicators with great diagnosis value
生物标志物 AUC(95% CI)值 P值 灵敏度(%) 特异度(%) 约登指数 皮损组与非皮损组 SP5 0.689(0.588~0.790) 0.001 73.0 61.0 0.340 RPL7A 0.689(0.589~0.789) < 0.001 75.0 59.3 0.343 SNRPC 0.684(0.583~0.784) < 0.001 68.8 62.7 0.315 LN组与非LN组 SP5 0.676(0.574~0.778) 0.002 74.1 55.1 0.292 RPL7A 0.664(0.560~0.767) 0.004 60.3 69.4 0.297 RPL35 0.663(0.559~0.766) 0.004 44.8 83.7 0.285 MAK16 0.652(0.548~0.756) 0.007 44.8 85.7 0.305 表 5 诊断价值较高的指标联合诊断结果
Table 5. Results in combined indicators with great diagnosis value
指标 AUC(95% CI)值 P值 灵敏度(%) 特异度(%) 约登指数 皮损组与非皮损组 RPL7A+SNRPC 0.706(0.608~0.803) < 0.001 54.2 81.3 0.355 SP5+RPL7A 0.701(0.601~0.800) < 0.001 59.3 81.3 0.406 SP5+SNRPC 0.689(0.589~0.788) 0.001 49.2 83.3 0.325 SP5+RPL7A+SNRPC 0.702(0.604~0.800) < 0.001 83.1 52.1 0.352 LN组与非LN组 SP5+RPL35 0.693(0.592~0.794) 0.001 70.7 65.3 0.360 SP5+RPL7A 0.688(0.586~0.789) 0.001 70.7 63.3 0.340 SP5+MAK16 0.683(0.581~0.784) 0.001 69.0 63.3 0.323 RPL35+MAK16 0.654(0.551~0.758) 0.006 46.6 83.7 0.303 RPL7A+RPL35 0.635(0.530~0.741) 0.016 79.3 47.0 0.263 RPL7A+MAK16 0.625(0.519~0.730) 0.027 63.8 63.3 0.271 SP5+RPL35+MAK16 0.684(0.583~0.786) 0.001 72.4 61.2 0.336 SP5+RPL7A+RPL35 0.679(0.577~0.781) 0.001 86.2 46.9 0.331 SP5+RPL7A+MAK16 0.673(0.571~0.775) 0.002 53.5 79.6 0.331 RPL7A+RPL35+MAK16 0.612(0.506~0.718) 0.046 32.8 89.8 0.226 SP5+RPL7A+RPL35+MAK16 0.661(0.558~0.764) 0.004 55.2 75.5 0.307 -
[1] Cozzani E, Drosera M, Gasparini G, et al. Serology of lupus erythematosus: correlation between immunopathological features and clinical aspects[J]. Autoimmune Dis, 2014(2014): 321359. DOI: 10.1155/2014/321359. [2] Ferreira TAR, de Andrade HM, de Pádua PM, et al. Identification of potential biomarkers for systemic lupus erythematosus diagnosis using two-dimensional differential gel electrophoresis (2D-DIGE) and mass spectrometry[J]. Autoimmunity, 2017, 50(4): 247-256. DOI: 10.1080/08916934.2017.1344975. [3] Kazemipour N, Qazizadeh H, Sepehrimanesh M, et al. Biomarkers identified from serum proteomic analysis for the differential diagnosis of systemic lupus erythematosus[J]. Lupus, 2015, 24(6): 582-587. DOI: 10.1177/0961203314558860. [4] Wu T, Ding H, Han J, et al. Antibody-array-based proteomic screening of serum markers in systemic lupus erythematosus: a discovery study[J]. J Proteome Res, 2016, 15(7): 2102-2114. DOI: 10.1021/acs.jproteome.5b00905. [5] Qi S, Chen Q, Xu D, et al. Clinical application of protein biomarkers in lupus erythematosus and lupus nephritis[J]. Lupus, 2018, 27(10): 1582-1590. DOI: 10.1177/0961203318773643. [6] Tedeschi SK, Johnson SR, Boumpas D, et al. Developing and refining new candidate criteria for systemic lupus erythematosus classification: an international collaboration[J]. Arthritis Care Res (Hoboken), 2018, 70(4): 571-581. DOI: 10.1002/acr.23317. [7] Sinha AA, Dey-Rao R. Genomic investigation of lupus in the skin[J]. J Investig Dermatol Symp Proc, 2017, 18(2): 75-80. DOI: 10.1016/j.jisp.2016.09.002. [8] Larosa M, laccarino L, Gatto M, et al. Advances in the diagnosis and classification of systemic lupus erythematosus[J]. Expert Rev Clin Immunol, 2016, 12(12): 1309-1320. DOI: 10.1080/1744666X.2016.1206470. [9] Ling HZ, Xu SZ, Leng RX, et al. Discovery of new serum biomarker panels for systemic lupus erythematosus diagnosis[J]. Rheumatology (Oxford), 2020, 59(6): 1416-1425. DOI: 10.1093/rheumatology/kez634. [10] Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus[J]. Arthritis Rheum, 1997, 40(9): 1725. DOI: 10.1002/art.1780400928. [11] Grankvist K, Gomez R, Nybo M, et al. Preanalytical aspects on short- and long-term storage of serum and plasma[J]. Diagnosis (Berl), 2019, 6(1): 51-56. DOI: 10.1515/dx-2018-0037. [12] Zhang YP, Wu J, Han YF, et al. Pathogenesis of cutaneous lupus erythema associated with and without systemic lupus erythema[J]. Autoimmun Rev, 2017, 16(7): 735-742. DOI: 10.1016/j.autrev.2017.05.009. [13] Misra R, Gupta R. Biomarkers in lupus nephritis[J]. Int J Rheum Dis, 2015, 18(2): 219-232. DOI: 10.1111/1756-185X.12602. [14] Esdaile JM, Abrahamowicz M, Joseph L, et al. Laboratory tests as predictors of disease exacerbations in systemic lupus erythematosus, Why some tests fail[J]. Arthritis Rheum, 1996, 39(3): 370-378. DOI: 10.1002/art.1780390304. [15] Julkunen H, Ekblom-Kullberg S, Miettinen A. Nonrenal and renal activity of systemic lupus erythematosus: a comparison of two anti-C1q and five anti-dsDNA assays and complement C3 and C4[J]. Rheumatol Int, 2012, 32(8): 2445-2451. DOI: 10.1007/s00296-011-1962-3. [16] Suske G, Bruford E, Philipsen S. Mammalian SP/KLF transcription factors: bring in the family[J]. Genomics, 2005, 85(5): 551-556. DOI: 10.1016/j.ygeno.2005.01.005. [17] Kennedy MW, Chalamalasetty RB, Thomas S, et al. Sp5 and Sp8 recruit beta-catenin and Tcf1-Lef1 to select enhancers to activate Wnt target gene transcription[J]. Proc Natl Acad Sci USA, 2016, 113(13): 3545-3550. DOI: 10.1073/pnas.1519994113. [18] Sfikakis PP, Boletis JN, Tsokos GC. Rituximab anti-B-cell therapy in systemic lupus erythematosus: pointing to the future[J]. Curr Opin Rheumatol, 2005, 17(5): 550-557. DOI: 10.1097/01.bor.0000172798.26249.fc. [19] Fu J, Hsu W. Epidermal Wnt controls hair follicle induction by orchestrating dynamic signaling crosstalk between the epidermis and dermis[J]. J Invest Dermatol, 2013, 133(4): 890-898. DOI: 10.1038/jid.2012.407. [20] Gudjonsson JE, Johnston A, Stoll SW, et al. Evidence for altered Wnt signaling in psoriatic skin[J]. J Invest Dermatol, 2010, 130(7): 1849-1859. DOI: 10.1038/jid.2010.67. [21] Tveita A, Rekvig OP, Zykova SN. Glomerular matrix Metalloproteinases and their regulators in the pathogenesis of lupus nephritis[J]. Arthritis Res Ther, 2008, 10(6): 229. DOI: 10.1186/ar2532. [22] He W, Dai C, Li Y, et al. Wnt/beta-catenin signaling promotes renal interstitial fibrosis[J]. J Am Soc Nephrol, 2009, 20(4): 765-776. DOI: 10.1681/ASN.2008060566. [23] Szodoray P, Nakken B, Barath S, et al. Altered Th17 cells and Th17/regulatory T-cell ratios indicate the subsequent conversion from undifferentiated connective tissue disease to definitive systemic autoimmune disorders[J]. Hum Immunol, 2013, 74(12): 1510-1518. DOI: 10.1016/j.humimm.2013.08.003. [24] Wang XD, Huang XF, Yan QR, et al. Aberrant activation of the WNT/beta-catenin signaling pathway in lupus nephritis[J]. PLoS One, 2014, 9(1): e84852. DOI: 10.1371/journal.pone.0084852. -





 下载:
下载: