A prospective cohort study on the relationship between hyperuricemia and fatty liver
-
摘要:
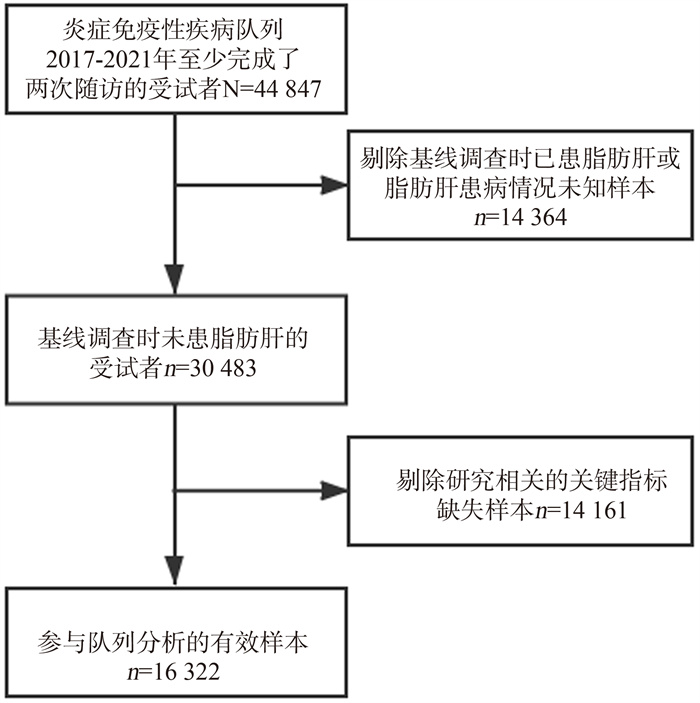
目的 通过前瞻性队列研究分析高尿酸血症(hyperuricemia, HUA)与脂肪性肝病(简称脂肪肝)发病风险的关系,为脂肪肝的防治提供依据。 方法 基于炎症免疫性疾病队列,纳入2017年―2021年至少完成了两次随访、与研究相关的关键指标无缺失且基线调查时未患脂肪肝的16 322名受试者作为本次研究对象。采用Cox比例风险回归模型分析HUA与脂肪肝发病风险之间的关联关系。模型1为单因素Cox分析;模型2调整年龄、性别和BMI;模型3在模型2的基础上调整高血糖、高血压、尿素氮、肌酐、总胆红素、AST、ALT和血脂异常。 结果 研究对象共随访42 472.75人年,平均随访时间2.60年,队列中新发脂肪肝病例3 150例,总人群发病密度为74.17/1 000人年。Cox比例风险回归模型分析结果显示,HUA组脂肪肝发病风险较高。3个模型风险比(hazard ration, HR)分别为2.049(95% CI: 1.861~2.256)、1.360(95% CI: 1.233~1.501)和2.049(95% CI: 1.861~2.256)。在对高血糖、高血压、血脂异常等因素分层后,HUA与脂肪肝发病风险仍呈正相关(均有P < 0.05)。交互作用研究显示:BMI(P=0.003)、高血压(P=0.012)与HUA存在相乘交互作用。 结论 HUA是脂肪肝发生的独立危险因素,及时干预HUA可以降低脂肪肝的发生或延缓脂肪肝的进展,为临床预防和治疗脂肪肝提供了参考依据。 -
关键词:
- 高尿酸血症 /
- 脂肪肝 /
- 队列研究 /
- Cox比例风险回归模型
Abstract:Objective The study aims to probe the relationship between hyperuricemia (HUA) and the risk of the fatty liver through a prospective cohort study, so as to provide the basis for the prevention and treatment of fatty liver. Methods Based on the Inflammatory and Immune Mediated Diseases Cohort, a total of 16 322 subjects who had completed at least two follow-ups from 2017 to 2021, had no missing key indicators related to the study and fatty liver at baseline were investigated. Cox proportional hazard model was used to analyze the relationship between HUA and the risk of fatty liver. Model 1 was a univariate Cox analysis, model 2 adjusted age, sex and BMI, and model 3 further adjusted hyperglycemia, hypertension, urea nitrogen, creatinine, total bilirubin, AST, ALT and dyslipidemia. Results The subjects were followed up for 42 472.75 person-years, with an average follow-up time of 2.60 years. There were 3 150 incidence cases of fatty liver, with an incidence density of 74.17/1 000 person-years. Cox proportional hazard model analysis showed that the risk of fatty liver was higher in HUA group. The hazard ration(HR) of the three models were 2.049(95% CI: 1.861-2.256), 1.360(95% CI: 1.233-1.501) and 2.049(95% CI: 1.861-2.256) respectively. After stratification of hyperglycemia, hypertension, dyslipidemia and other factors, HUA was still positively associated with the risk of fatty liver (all P < 0.05). In the multiplication model, significant multiplier interactions were found between BMI (P=0.003), hypertension (P=0.012) and HUA. Conclusions HUA is an independent risk factor for fatty liver. The timely intervention of HUA can reduce the occurrence of fatty liver or delay the progress of fatty liver. Our study provides reference for the clinical prevention and treatment of fatty liver. -
Key words:
- Hyperuricemia /
- Fatty liver /
- Cohort study /
- Cox proportional hazard model
-
表 1 研究对象的基线特征[M(P25, P75)]
Table 1. Baseline characteristics of the study participants [M(P25, P75)]
变量 合计(N=16 322) 有无脂肪肝 Z/χ2值 P值 无(n=13 172) 有(n=3 150) 年龄(岁) 44(33, 55) 43(33, 54) 48(37, 57) -12.851 < 0.001 性别 733.980 < 0.001 女 8 083(49.52) 7 206(54.71) 877(27.84) 男 8 239(50.48) 5 966(45.29) 2 273(72.16) BMI(kg/m2) 1 036.440 < 0.001 <18.5 1 108(6.79) 1 080(8.20) 28(0.89) 18.5~<24.0 11 204(68.64) 9 496(72.09) 1 708(54.22) 24.0~<28.0 3 674(22.51) 2 433(18.47) 1 241(39.40) ≥28.0 336(2.06) 163(1.24) 173(5.49) SBP(mm Hg) 122(112, 135) 121(111, 134) 128(117, 139) -18.630 < 0.001 DBP(mm Hg) 74(67, 82) 73(67, 81) 78(71, 86) -19.481 < 0.001 FPG(mmol/L) 5.03(4.75, 5.38) 5.01(4.73, 5.35) 5.14(4.84, 5.54) -13.574 < 0.001 SUA(μmol/L) 296(247, 351) 287(240, 340) 334(286, 386) -29.188 < 0.001 HDL-C(mmol/L) 1.44(1.21, 1.72) 1.49(1.26, 1.76) 1.27(1.08, 1.49) -30.339 < 0.001 LDL-C(mmol/L) 2.58(2.12, 3.11) 2.54(2.09, 3.06) 2.76(2.29, 3.29) -14.800 < 0.001 TC(mmol/L) 4.52(4.00, 5.13) 4.49(3.97, 5.09) 4.67(4.13, 5.29) -10.483 < 0.001 TG(mmol/L) 1.07(0.79, 1.48) 1.00(0.76, 1.37) 1.41(1.03, 1.93) -34.369 < 0.001 Scr(μmol/L) 69(58, 81) 67(57, 80) 75(64, 84) -19.945 < 0.001 UN(mmol/L) 4.78(4.03, 5.67) 4.73(3.99, 5.60) 4.99(4.20, 5.93) -10.701 < 0.001 TBIL(μmol/L) 12.90(10.30, 16.20) 12.80(10.30, 16.20) 12.90(10.20, 16.30) -0.204 0.838 ALT(U/L) 17.00(14.00, 23.00) 17.00(13.00, 22.00) 21.00(16.00, 28.00) -26.913 < 0.001 AST(U/L) 18.00(16.00, 22.00) 18.00(16.00, 22.00) 19.00(17.00, 23.00) -13.614 < 0.001 高血糖 103.804 < 0.001 否 15 198(93.11) 12 395(94.10) 2 803(88.98) 是 1 124(6.89) 777(5.90) 347(11.02) 高血压 149.453 < 0.001 否 12 906(79.07) 10 666(80.97) 2 240(71.11) 是 3 416(20.93) 2 506(19.03) 910(28.89) 血脂异常 511.308 < 0.001 否 10 292(63.06) 8 856(67.23) 1 436(45.59) 是 6 030(36.94) 4 316(32.77) 1 714(54.41) 高尿酸血症 206.796 < 0.001 否 14 853(91.00) 12 194(92.58) 2 659(84.41) 是 1 469(9.00) 978(7.42) 491(15.59) 表 2 HUA与脂肪肝发病风险的关联[HR(95% CI)]
Table 2. Association between HUA and fatty liver disease [HR(95% CI)]
变量 模型1 模型2 模型3 变量 模型1 模型2 模型3 SUA分组 UN(mmol/L) 1.027(0.997~1.058) 非HUA组 1.000 1.000 1.000 TBIL(μmol/L) 0.989(0.982~0.996) b HUA组 2.049(1.861~2.256) a 1.360(1.233~1.501) a 1.353(1.222~1.497) a ALT(U/L) 1.012(1.009~1.015) a 年龄(岁) 1.005(1.003~1.008) a 1.004(1.002~1.007) b AST(U/L) 0.985(0.980~0.990) a 性别 高血糖 女 1.000 1.000 否 1.000 男 2.195(2.025~2.378) a 2.499(2.252~2.773) a 是 1.194(1.060~1.345) b BMI(kg/m2) 高血压 18.5~<24.0 1.000 1.000 否 1.000 <18.5 0.182(0.125~0.265) a 0.202(0.139~0.294) a 是 1.059(0.973~1.153) 24.0~<28.0 2.020(1.874~2.178) a 1.834(1.699~1.979) a 血脂异常 ≥28.0 3.421(2.922~4.005) a 2.893(2.467~3.394) a 否 1.000 Scr(μmol/L) 0.991(0.988~0.995) a 是 1.719(1.599~1.847) a 注:a表示P < 0.001; b表示P < 0.05。 表 3 HUA与脂肪肝发病风险的亚组分析和相乘交互作用分析
Table 3. Subgroup analysis and multiplicative interaction effect of HUA and risk of fatty liver disease
变量 例数/总例数 多因素Cox分析 P相乘交互作用值 HR (95% CI) 值 P值 BMI(kg/m2) 0.003 <18.5 1 108/16 322 1.386(0.302~6.364) 0.675 18.5~<24.0 11 204/16 322 1.582(1.367~1.832) < 0.001 24.0~<28.0 3 674/16 322 1.180(1.014~1.373) 0.032 ≥28.0 336/16 322 1.047(0.711~1.541) 0.815 高血糖 0.495 否 15 198/16 322 1.344(1.207~1.498) < 0.001 是 1 124/16 322 1.392(1.033~1.875) 0.030 高血压 0.012 否 12 906/16 322 1.446(1.277~1.638) < 0.001 是 3 416/16 322 1.231(1.034~1.466) 0.019 血脂异常 0.668 否 10 292/16 322 1.325(1.115~1.575) 0.001 是 6 030/16 322 1.375(1.212~1.559) < 0.001 注:各模型均调整其余变量(年龄、性别、BMI、Scr、UN、TBIL、ALT、AST、高血糖、高血压和血脂异常)。 表 4 HUA与脂肪肝发病风险的关联a
Table 4. Association between HUA and risk of fatty liver disease a
变量 模型1 模型2 模型3 SUA分组 非HUA组 1.000 1.000 1.000 HUA组 2.171(1.959~2.405) b 1.275(1.147~1.417) b 1.260(1.130~1.405) b 年龄(岁) 1.005(1.003~1.008) b 1.004(1.002~1.007) c 性别 女 1.000 1.000 男 2.183(2.012~2.368) b 2.456(2.214~2.725) b BMI(kg/m2) 18.5~ < 24.0 1.000 1.000 < 18.5 0.181(0.125~0.263) b 0.201(0.139~0.293) b 24.0~ < 28.0 2.033(1.886~2.191) b 1.843(1.708~1.988) b ≥28.0 3.464(2.959~4.055) b 2.920(2.490~3.426) b Scr(μmol/L) 0.992(0.989~0.995) b UN(mmol/L) 1.027(0.997~1.058) TBIL(μmol/L) 0.989(0.983~0.996) c ALT(U/L) 1.012(1.009~1.014) b AST(U/L) 0.985(0.980~0.991) b 高血糖 否 1.000 是 1.194(1.060~1.345) c 高血压 否 1.000 是 1.064(0.978~1.159) 血脂异常 否 1.000 是 1.728(1.607~1.857) b 注:a表示根据SUA>420 μmol/L即为HUA的标准对SUA分组进行的Cox分析。模型1为单因素Cox分析,模型2调整了年龄、性别和BMI,模型3调整了年龄、性别、BMI、Scr、UN、TBIL、ALT、AST、高血糖、高血压和血脂异常; b表示P < 0.001; c表示P < 0.05。 -
[1] Seidell JC. Obesity, insulin resistance and diabetes--a worldwide epidemic[J]. Br J Nutr, 2000, 83 (Suppl 1): S5-S8. DOI: 10.1017/s000711450000088x. [2] Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010[J]. Lancet, 2012, 380(9859): 2095-2128. DOI: 10.1016/S0140-6736(12)61728-0. [3] Socha P, Wierzbicka A, Neuhoff-Murawska J, et al. Nonalcoholic fatty liver disease as a feature of the metabolic syndrome[J]. Rocz Panstw Zakl Hig, 2007, 58(1): 129-137. [4] Fabbrini E, Sullivan S, Klein S. Obesity and nonalcoholic fatty liver disease: biochemical, metabolic, and clinical implications[J]. Hepatology, 2010, 51(2): 679-689. DOI: 10.1002/hep.23280. [5] Hamaguchi M, Kojima T, Takeda N, et al. The metabolic syndrome as a predictor of nonalcoholic fatty liver disease[J]. Ann Intern Med, 2005, 143(10): 722-728. DOI: 10.7326/0003-4819-143-10-200511150-00009. [6] Hediger MA, Johnson RJ, Miyazaki H, et al. Molecular physiology of urate transport[J]. Physiology(Bethesda), 2005, 20: 125-133. DOI: 10.1152/physiol.00039.2004. [7] Edwards NL. The role of hyperuricemia in vascular disorders[J]. Curr Opin Rheumatol, 2009, 21(2): 132-137. DOI: 10.1097/BOR.0b013e3283257b96. [8] Liu R, Han C, Wu D, et al. Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014: a systematic review and meta-analysis[J]. Biomed Res Int, 2015, 2015: 762820. DOI: 10.1155/2015/762820. [9] Sun DQ, Wu SJ, Liu WY, et al. Serum uric acid: a new therapeutic target for nonalcoholic fatty liver disease[J]. Expert Opin Ther Targets, 2016, 20(3): 375-387. DOI: 10.1517/14728222.2016.1096930. [10] Baba T, Amasaki Y, Soda M, et al. Fatty liver and uric acid levels predict incident coronary heart disease but not stroke among atomic bomb survivors in Nagasaki[J]. Hypertens Res, 2007, 30(9): 823-829. DOI: 10.1291/hypres.30.823. [11] Li S, Fu Y, Liu Y, et al. Serum uric acid levels and nonalcoholic fatty liver disease: a 2-sample bidirectional mendelian randomization study[J]. J Clin Endocri Metab, 2022, 107(8): e3497-e3503. DOI: 10.1210/clinem/dgac190. [12] Sirota JC, McFann K, Targher G, et al. Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: liver ultrasound data from the National Health and Nutrition Examination Survey[J]. Metabolism, 2013, 62(3): 392-399. DOI: 10.1016/j.metabol.2012.08.013. [13] Hwang IC, Suh SY, Suh AR, et al. The relationship between normal serum uric acid and nonalcoholic fatty liver disease[J]. J Korean Med Sci, 2011, 26(3): 386-391. DOI: 10.3346/jkms.2011.26.3.386. [14] Xie Y, Wang M, Zhang Y, et al. Serum uric acid and non-alcoholic fatty liver disease in non-diabetic Chinese men[J]. PLoS One, 2013, 8(7): e67152. DOI: 10.1371/journal.pone.0067152. [15] McCullough AJ. The clinical features, diagnosis and natural history of nonalcoholic fatty liver disease[J]. Clin Liver Dis, 2004, 8(3): 521-533. DOI: 10.1016/j.cld.2004.04.004. [16] 中华医学会内分泌学分会. 中国高尿酸血症与痛风诊疗指南(2019)[J]. 中华内分泌代谢杂志, 2020, 36(1): 1-13. DOI: 10.3760/cma.j.issn.1000-6699.2020.01.001.Chinese Society of Endocrinology, Chinese Medical Association. Guideline for the diagnosis and treatment of hyperuricemia and gout in China (2019)[J]. Chin J Endocrinol Metab, 2020, 36(1): 1-13. DOI: 10.3760/cma.j.issn.1000-6699.2020.01.001. [17] WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies[J]. Lancet, 2004, 363(9403): 157-163. DOI: 10.1016/S0140-6736(03)15268-3. [18] 中华医学会糖尿病分会. 中国2型糖尿病防治指南(2017年版)[J]. 中国实用内科杂志, 2018, 38(4): 292-344. DOI: 10.19538/j.nk2018040108.Diabetes Branch of Chinese Medical Association. Guideline for the prevention and treatment of type 2 diabetes in China (2017 Edition)[J]. Chin J Pract Intern Med, 2018, 10(1): 4-67. DOI: 10.19538/j.nk2018040108. [19] 中国高血压防治指南修订委员会, 高血压联盟(中国), 中华医学会心血管病学分会, 等. 中国高血压防治指南(2018年修订版)[J]. 中国心血管杂志, 2019, 24(1): 24-56. DOI: 10.3969/j.issn.1007-5410.2019.01.002.Writing Group of 2018 Chinese Guidelines for the Management of Hypertension, Chinese Hypertension League, Chinese Society of Cardiology, et al. Guideline for the prevention and treatment of hypertension in China (Revised Edition 2018)[J]. Chin J Cardiovasc Med, 2019, 24(1): 24-56. DOI: 10.3969/j.issn.1007-5410.2019.01.002. [20] 中国成人血脂异常防治指南修订联合委员会. 中国成人血脂异常防治指南(2016年修订版)[J]. 中国循环杂志, 2016, 31(10): 937-953. DOI: 10.3969/j.issn.1000-3614.2016.10.001.Joint Committee for Revision of Guidelines for the Prevention and Treatment of Adult Dyslipidemia in China. Guideline for the prevention and treatment of adult dyslipidemia in China (Revised Edition 2016)[J]. Chin Circul J, 2016, 31(10): 937-953. DOI: 10.3969/j.issn.1000-3614.2016.10.001. [21] Ryu S, Chang Y, Kim SG, et al. Serum uric acid levels predict incident nonalcoholic fatty liver disease in healthy Korean men[J]. Metabolism, 2011, 60(6): 860-866. DOI: 10.1016/j.metabol.2010.08.005. [22] Wu SJ, Zhu GQ, Ye BZ, et al. Association between sex-specific serum uric acid and non-alcoholic fatty liver disease in Chinese adults: a large population-based study[J]. Medicine(Baltimore), 2015, 94(17): e802. DOI: 10.1097/MD.0000000000000802. [23] Yamada T, Suzuki S, Fukatsu M, et al. Elevated serum uric acid is an independent risk factor for nonalcoholic fatty liver disease in Japanese undergoing a health checkup[J]. Acta Gastroentero Belgica, 2010, 73(1): 12-17. [24] Xu CF, Yu CH, Xu L, et al. High serum uric acid increases the risk for nonalcoholic Fatty liver disease: a prospective observational study[J]. PLoS One, 2010, 5(7): e11578. DOI: 10.1371/journal.pone.0011578. [25] Li C, Hsieh MC, Chang SJ. Metabolic syndrome, diabetes, and hyperuricemia[J]. Curr Opin Rheumatol, 2013, 25(2): 210-216. DOI: 10.1097/BOR.0b013e32835d951e. [26] Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis[J]. Nat Rev Gastroenterol Hepatol, 2013, 10(6): 330-344. DOI: 10.1038/nrgastro.2013.41. [27] Lanaspa MA, Sanchez-Lozada LG, Cicerchi C, et al. Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver[J]. PLoS One, 2012, 7(10): e47948. DOI: 10.1371/journal.pone.0047948. [28] Lanaspa MA, Sanchez-Lozada LG, Choi YJ, et al. Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver[J]. J Biol Chem, 2012, 287(48): 40732-40744. DOI: 10.1074/jbc.M112.399899. [29] Harrison R. Physiological roles of xanthine oxidoreductase[J]. Drug Metab Rev, 2004, 36(2): 363-375. DOI: 10.1081/dmr-120037569. [30] Xu CF, Wan XY, Xu L, et al. Xanthine oxidase in non-alcoholic fatty liver disease and hyperuricemia: one stone hits two birds[J]. J Hepatol, 2015, 62(6): 1412-1419. DOI: 10.1016/j.jhep.2015.01.019. [31] Wan XY, Xu CF, Lin YM, et al. Uric acid regulates hepatic steatosis and insulin resistance through the NLRP3 inflammasome-dependent mechanism[J]. J Hepatol, 2016, 64(4): 925-932. DOI: 10.1016/j.jhep.2015.11.022. [32] Hernaez R, Lazo M, Bonekamp S, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis[J]. Hepatology, 2011, 54(3): 1082-1090. DOI: 10.1002/hep.24452. -





 下载:
下载: