The highly active antiretroviral therapy effect among HIV/AIDS patients with and without HCV coinfection in Chengdu City
-
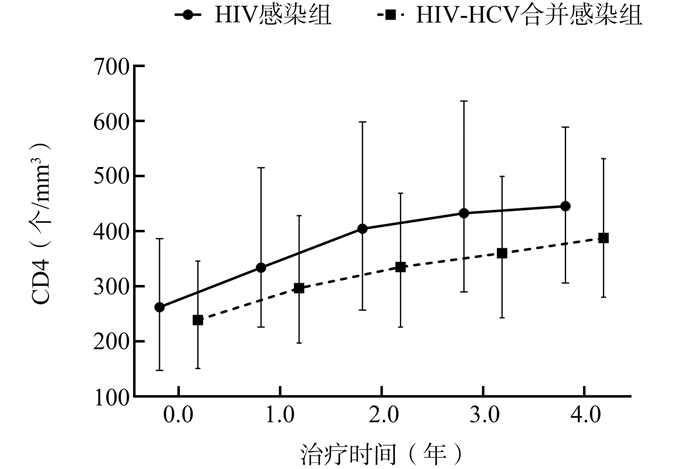
摘要:
目的 了解艾滋病病毒(human immunodeficiency virus, HIV)-丙型肝炎病毒(hepatitis C virus, HCV)合并感染者和HIV感染者艾滋病(acquired immunedeficiency syndrome, AIDS)抗病毒治疗(Antiretroviral therapy, ART)的效果,并进一步分析HCV合并感染对艾滋病ART效果的影响。 方法 利用国家艾滋病综合防治信息系统选取成都市2010年1月1日至2018年12月31日确诊的HIV/AIDS病例信息,随访时间截至2019年12月31日。采用Cox比例风险模型分析HCV感染对艾滋病ART病毒学失败的影响。 结果 符合纳入标准的HIV-HCV合并感染者共555例,另匹配HIV感染者555例。在接受艾滋病抗病毒治疗4年内,HIV感染组和HIV-HCV合并感染组CD4+T淋巴细胞计数(简称CD4)的中位数年均分别升高45.9个/mm3、37.1个/mm3。两组病例累计随访2 393.1人年,病毒学失败率为7.15/100人年(171/2 393.1)。多因素Cox分析结果显示,HIV-HCV合并感染组发生病毒学失败的风险是HIV感染组的1.512(1.042~2.195)倍(P=0.030)。 结论 合并HCV感染可能是影响HIV/AIDS艾滋病抗病毒治疗效果的危险因素。 Abstract:Objective This study aimed to analyze the impact of hepatitis C virus (HCV) coinfection on antiretroviral therapy(ART) effect with human immunodeficiency virus (HIV), and examine the impact of HCV coinfection on CD4+T cells count (CD4) and virologic failure (VF) in patients with HIV. Methods All data were extracted from the National HIV/AIDS (human immunodeficiency virus /acquired immunodeficiency syndrome) Comprehensive Response Information Management System. Information were collected from HIV-HCV coinfection patients who diagnosed with HIV during 2010-2018, with follow-up conducted till December 31, 2019. Cox proportional hazard model was used to analyze the factors associated with VF after ART. Results 555 HIV-HCV coinfection patients and matched another 555 HIV monoinfection patients were included. An increased CD4 cell count was observed in all groups within 4 years of receiving ART treatment, the average annual increase in the HIV monoinfection group and HIV-HCV co-infection group were 45.9 cells/mm3 and 37.1 cells/mm3 for the median CD4 cell counts. 171 individuals experienced VF with an incidence rate of 7.15 per 100 person-years. The multivariate Cox proportional hazard analysis showed that the HIV-HCV coinfection group was associated with increased hazard of VF (aHR=1.512, 95% CI: 1.042-2.195, P=0.030) compared to HIV monoinfection group. Conclusion HIV-HCV coinfection was significantly associated with increased hazard of VF during highly active antiretroviral therapy for HIV/AIDS. -
Key words:
- HIV /
- HCV /
- Antiretroviral Therapy /
- Virologic failure
-
表 1 HIV-HCV合并感染组与HIV感染组的基本情况
Table 1. Baseline characteristics of HIV-HCV co-infection and HIV monoinfection groups
变量 HIV感染组 HIV-HCV合并感染组 t/χ2值 P值 HIV确诊时年龄[岁,(x±s)] 39.66±14.26 40.63±13.54 1.161 0.246 婚姻状况 2.567 0.463 未婚 194 169 已婚或同居 207 222 离异/分居/丧偶 144 153 不详 10 11 传播途径 159.67 < 0.001 注射吸毒或采(输)血传播 8 138 同性性传播 147 61 异性性传播 388 332 不详或其他 12 24 HIV确诊到治疗时间(d) 46.419 < 0.001 0~ 145 155 31~ 124 215 ≥366 286 185 WHO临床分期 4.160 0.245 Ⅰ 231 202 Ⅱ 185 201 Ⅲ 99 116 Ⅳ 40 36 ALT (U/L) 44.371 < 0.001 0~ 424 320 ≥40 124 219 缺失 7 16 AST(U/L) 95.055 < 0.001 0~ 464 316 ≥40 81 204 缺失 10 35 基线CD4(个/mm3) 11.758 0.019 0~ 196 212 200~ 178 206 350~ 100 84 ≥500 79 49 缺失 2 4 初始治疗方案 55.268 < 0.001 TDF+3TC+EFV/NVP 496 412 AZT/D4T+3TC+EFV/NVP 35 121 其他 24 22 注:ALT:丙氨酸氨基转移酶;AST:天门冬氨酸氨基转移酶;TDF:替诺福韦;3TC:拉米夫定;AZT:齐多夫定;D4T:司他夫定;NVP:奈韦拉平;EFV:依非韦伦。 表 2 成都市HIV/AIDS抗病毒治疗病毒学失败的影响因素分析
Table 2. The multiple factors analysis for Cox regression model of virologic failure among HIV/AIDS patients on ART in Chengdu City
变量 β sx Wald值 aHR (95% CI)值 P值 基线CD4(个/mm3) 0~ 1.000 0.008 200~ -0.421 0.182 5.350 0.656(0.460~0.938) 0.021 350~ -0.258 0.236 1.197 0.772(0.486~1.227) 0.274 ≥500 -1.108 0.377 8.644 0.330(0.158~0.691) 0.003 AST(U/L) 0~ 1.000 ≥40 0.219 0.217 1.015 1.245(0.813~1.905) 0.314 ALT(U/L) 0~ 1.000 ≥40 0.029 0.210 0.019 1.029(0.682~1.554) 0.890 HIV确诊到治疗时间(d) 0~ 1.000 0.689 31~ 0.159 0.209 0.580 1.173(0.778~1.768) 0.446 ≥366 0.115 0.212 0.293 1.121(0.741~1.698) 0.588 是否合并HCV感染 否 1.000 是 0.414 0.190 4.732 1.512(1.042~2.195) 0.030 初始治疗方案 TDF+3TC+EFV/NVP 1.000 其他方案 0.360 0.219 2.692 1.433(0.932~2.204) 0.101 传播途径 性传播与其他 1.000 注射吸毒或采(输)血传播 0.385 0.218 3.123 1.469(0.959~2.252) 0.077 注:ALT:丙氨酸氨基转移酶;AST:天门冬氨酸氨基转移酶;TDF:替诺福韦;3TC:拉米夫定;NVP:奈韦拉平;EFV:依非韦伦。 -
WHO. HIV/AIDS, Key facts[EB/OL]. (2019-07-25)[ 2020-04-12]. https://www.who.int/en/news-room/fact-sheets/detail/hiv-aids. WHO. Hepatitis C, Key facts[EB/OL]. (2019-07-09)[ 2020-04-12]. https://www.who.int/en/news-room/fact-sheets/detail/hepatitis-c. Platt L, Easterbrook P, Gower E, et al. Prevalence and burden of HCV co-infection in people living with HIV: a global systematic review and meta-analysis[J]. Lancet Infect Dis, 2016, 16(7):797-808. DOI: 10.1016/S1473-3099(15)00485-5. Benhammou V, Tubiana R, Matheron S, et al. HBV or HCV coinfection in HIV-1-infected pregnant women in France: prevalence and pregnancy outcomes[J]. J Acquir Immune Defic Syndr, 2018, 77(5):439-450. DOI: 10.1097/QAI.0000000000001618. Mena A, Meijide H, Rodríguez-Osorio I, et al. Liver-related mortality and hospitalizations attributable to chronic hepatitis C virus coinfection in persons living with HIV[J]. HIV Med, 2017, 18(9):685-689. DOI: 10.1111/hiv.12502. 豆智慧, 张福杰, 赵燕, 等. 2002-2014年中国免费艾滋病抗病毒治疗进展[J].中华流行病学杂志, 2015, 36(12):1345-1350. DOI: 10.3760/cma.j.issn.0254-6450.2015.12.005Dou ZH, Zhang FJ, Zhao Y, et al. Progress on China's national free antiretroviral therapy strategy in 2002-2014[J]. Chin J Epidemiol, 2015, 36(12):1345-1350. DOI: 10.3760/cma.j.issn.0254-6450.2015.12.005. Willis SJ, Cole SR, Westreich D, et al. Chronic hepatitis C virus infection and subsequent HIV viral load among women with HIV initiating antiretroviral therapy[J]. AIDS, 2018, 32(5):653-661. DOI: 10.1097/QAD.0000000000001745. 钟敏, 李娅, 何增品, 等.昆明地区HIV-HCV共感染人群基因型耐药突变的特点[J].昆明医科大学学报, 2019, 40(4):126-130. DOI: 10.3969/j.issn.1003-4706.2019.04.026.Zhong M, Li Y, He ZP, et al. Study on the Characteristics of genotypic drug resistance mutation in HIV-HCV co-infected population in Kunming[J]. Journal of Kunming Medical University, 2019, 40(4):126-130. DOI: 10.3969/j.issn.1003-4706.2019.04.026. Silva CMD, Peder LD, Silva ES, et al. Impact of HBV and HCV coinfection on CD4 cells among HIV-infected patients: a longitudinal retrospective study[J]. J Infect Dev Ctries, 2018, 12(11):1009-1018. DOI: 10.3855/jidc.10035. 国家卫生健康委员会.艾滋病和艾滋病病毒感染诊断: WS293-2019[S].北京: 中国标准出版社, 2019.National Health Commission of the People's Republic of China. Diagnosis for HIV/AIDS: WS293-2019[S]. Beijing: Standards Press of China, 2019. 中华医学会肝病学分会, 中华医学会感染病学分会.丙型肝炎防治指南(2015更新版)[J].中华肝脏病杂志, 2015, 23(12):906-923. DOI: 10.3760/cma.j.issn.1007-3418.2015.12.003.Chinese Society of Hepatology, Chinese Medical Association, Chinese Society of Infectious Diseases, Chinese Medical Association. The guideline of prevention and treatment for hepatitis C: a 2015 update[J]. Chin J Hepatol, 2015, 23(12):906-923. DOI: 10.3760/cma.j.issn.1007-3418.2015.12.003. Martinello M, Amin J, Matthews GV, et al. Prevalence and disease burden of HCV coinfection in HIV cohorts in the Asia Pacific Region: A systematic review and meta-analysis[J]. AIDS Rev, 2016, 18(2):68-80. 王继宝, 陈晓晨, 段星, 等.云南省德宏州2016-2017年新报告中国和缅甸籍HIV感染者中HCV的流行病学特征[J].中华疾病控制杂志, 2019, 23(10):1293-1296, 1300. DOI: 10.16462/j.cnki.zhjbkz.2019.10.026Wang JB, Chen XC, Duan X, et al. Epidemiological characteristics of co-infection of hepatitis C virus among newly reported HIV infected patients in Chinese and Burmese from 2016 to 2017 in Dehong Prefecture of Yunnan Province[J]. Chin J Dis Control Prev, 2019, 23(10):1293-1296, 1300. DOI: 10.16462/j.cnki.zhjbkz.2019.10.026. Schlabe S, Rockstroh JK. Advances in the treatment of HIV-HCV coinfection in adults[J]. Expert Opin Pharmacother, 2018, 19(1):49-64. DOI: 10.1080/14656566.2017.1419185. 葛琳, 李东民, 李培龙, 等. 2010-2015年中国艾滋病哨点监测人群HIV、梅毒和HCV感染状况分析[J].疾病监测, 2017, 32(2):111-117. DOI: 10.3784/j.issn.1003-9961.2017.02.008.Ge L, Li DM, Li PL, et al. Population specific sentinel surveillance for HIV infection, syphilis and HCV infection in China, during 2010-2015[J]. Dis Surveill, 2017, 32(2):111-117. DOI: 10.3784/j.issn.1003-9961.2017.02.008. -





 下载:
下载: