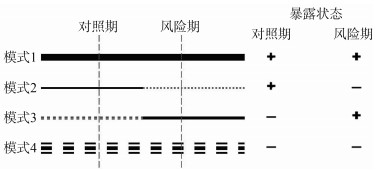
Methodological progress in selection of control in observation study in the context of big data
-
摘要: 对照选择作为流行病学研究设计的核心,伴随健康医疗大数据研究的日益增多,其策略在不断丰富和完善,不同策略潜在影响的估计方法也在不断提出和推广。中国流行病学工作者亟需紧跟国际对照选择相关的方法学趋势,发现并解决方法学本土化问题,以便更好地服务于健康医疗大数据的开发。Abstract: Control selection is the core of epidemiological study. With the increase of studies based on big data in healthcare, the strategies for control selection were continued to enrich and improve. The methodologies to estimate the potential impact of each control selection strategies were also proposed. To facilitate the utilization of big data in healthcare, the Chinese epidemiologist should keep in step with the international trend in the field of control selection, and should find ways to localize the strategies for control selection.
-
Key words:
- Control selection /
- Observational study /
- Big data in healthcare
-
表 1 RR对应的E值计算公式
Table 1. Relative risk (RR) corresponding E value calculation formula
研究中RR的大小 E值计算公式 RR>1 点值 $E=R R+\sqrt{R R \times(R R-1)}$ 置信区间 RR置信区间下限LL≤1,E = 1
RR置信区间下限$L L>1, E=L L+\sqrt{L L \times(L L-1)}$RR<1 点值 $E=\frac{1}{R R}+\sqrt{\frac{1}{R R} \times\left(\frac{1}{R R}-1\right)}$ 置信区间 RR置信区间上限UL≥1,E = 1
RR置信区间上限$U L<1, E=\frac{1}{U L}+\sqrt{\frac{1}{U L} \times\left(\frac{1}{U L}-1\right)}$注:RR,risk ratio,比值比; LL,lower limit,下线; UL,upper limit,上限。 -
[1] 张政, 詹思延.病例交叉设计[J].中华流行病学杂志, 2001, 22(4):304-306.Zhang Z, Zhan SY. Case crossover design[J]. Chin J Epidemiol, 2001, 22(4):304-306. [2] 王胜锋, 詹思延.大数据时代的药品安全主动监测:对照选择的挑战与机遇[J].中华流行病学杂志, 2016, 37(7):909-916. DOI: 10.3760/cma.j.issn.0254-6450.2016.07.001.Wang SF, Zhan SY. Active surveillance of adverse drug reaction in the era of big data: challenge and opportunity for control selection[J]. Chin J Epidemiol, 2016, 37(7):909-916. DOI: 10.3760/cma.j.issn.0254-6450.2016.07.001. [3] 中国药学会药物流行病学专业委员会.中国药物流行病学研究方法学指南(T/CPHARMA 002-2019)[J].中华流行病学杂志, 2019, 40(10):1180-1185. DOI: 10.3760/cma.j.issn.0254-6450.2019.10.002.Pharmacoepidemiology Professional Committee of Chinese Pharmaceutical Association. Guide on methodological standards in pharmacoepidemiology (T/CPHARMA 002-2019)[J]. Chin J Epidemiol, 2019, 40(10):1180-1185. DOI: 10.3760/cma.j.issn.0254-6450.2019.10.002. [4] Corrigan-Curay J, Sacks L, Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness[J]. JAMA, 2018, 320(9):867-868. DOI: 10.1001/jama.2018.10136. [5] Toh S. Analytic and data sharing options in real-world multidatabase studies of comparative effectiveness and safety of medical products[J]. Clin Pharmacol Ther, 2020, 107(4):834-842. DOI: 10.1002/cpt.1754. [6] Weinberg CR. Invited commentary: self-control is a virtue[J]. Am J Epidemiol, 2017, 185(11):1184-1186. DOI: 10.1093/aje/kwx075. [7] Gault N, Castañeda-Sanabria J, De Rycke Y, et al. Self-controlled designs in pharmacoepidemiology involving electronic healthcare databases: a systematic review[J]. BMC Med Res Methodol, 2017, 17(1):25. DOI: 10.1186/s12874-016-0278-0. [8] Hallas J, Pottegård A, Wang S, et al. Persistent user bias in case-crossover studies in pharmacoepidemiology[J]. Am J Epidemiol, 2016, 184(10):761-769. DOI: 10.1093/aje/kww079. [9] Bykov K, Wang SV, Hallas J, et al. Bias in case-crossover studies of medications due to persistent use: A simulation study[J]. Pharmacoepidemiol Drug Saf, 2020, 29(9):1079-1085. DOI: 10.1002/pds.5031. [10] Baker MA, Lieu TA, Li L, et al. A vaccine study design selection framework for the postlicensure rapid immunization safety monitoring program[J]. Am J Epidemiol, 2015, 181(8):608-618. DOI: 10.1093/aje/kwu322. [11] Schneeweiss S, Suissa S. Discussion of Schuemie et al: "A plea to stop using the case-control design in retrospective database studies"[J]. Stat Med, 2019, 38(22):4209-4212. DOI: 10.1002/sim.8320. [12] Connolly JG, Wang SV, Fuller CC, et al. Development and application of two semi-automated tools for targeted medical product surveillance in a distributed data network[J]. Curr Epidemiol Rep, 2017, 4(4):298-306. DOI: 10.1007/s40471-017-0121-0. [13] Gruber S, Chakravarty A, Heckbert SR, et al. Design and analysis choices for safety surveillance evaluations need to be tuned to the specifics of the hypothesized drug-outcome association[J]. Pharmacoepidemiol Drug Saf, 2016, 25(9):973-981. DOI: 10.1002/pds.4065. [14] VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value[J]. Ann Intern Med, 2017, 167(4):268-274. DOI: 10.7326/M16-2607. [15] Smith LH, VanderWeele TJ. Simple sensitivity analysis for control selection bias[J]. Epidemiology, 2020, 31(5):e44-e45. DOI: 10.1097/EDE.0000000000001207. -




 下载:
下载: