Analysis for characteristics of genetic mutations among multi-drug resistant Mycobacterium tuberculosis (MDR-TB) isolates from Zhejiang Province
-
摘要:
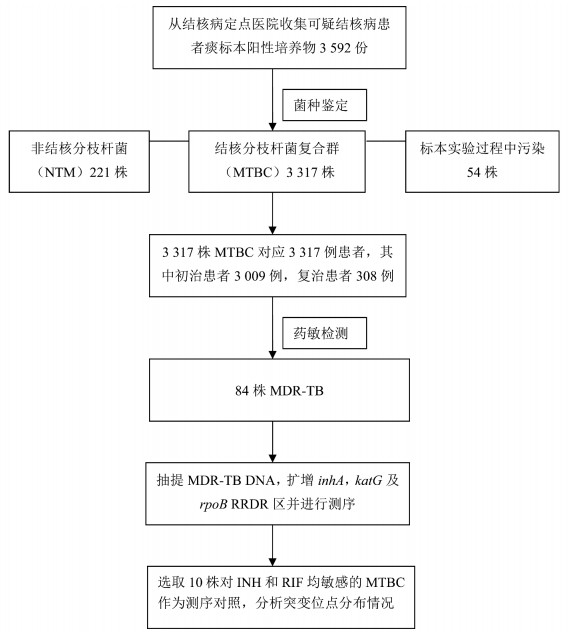
目的 分析浙江地区耐多药结核分枝杆菌(M.tuberculosis complex, MTBC)异烟肼(isoniazid, INH)和利福平(rifampicin, RIF)耐药相关基因突变特征。 方法 对患者痰标本阳性培养物进行菌种鉴定,提取耐多药菌株DNA,应用PCR法对inhA、katG和rpoB RRDR区进行扩增,并将扩增产物直接进行基因测序。 结果 共鉴定出3 317株MTBC,对应的患者中初治为3 009例,复治为308例,耐多药结核病(multidrug-resistant tuberculosis, MDR-TB)为84例,占比为2.53%(84/3 317)。其中,在INH耐药方面,47株分别在katG(315)或inhA(-15/-8)发生单位点突变,19株同时出现katG(315)和inhA(-15/-8)突变,18株未检测出上述基因突变,基因型同表型的符合率为78.57%(66/84),突变频率最高的位点是Ser315Thr,占47.62%(40/84)。在RIF耐药方面,79株在rpoB(511、512、513、516、522、526和531位点)出现突变,2株同时出现511/513和511/516突变,突变频率最高的位点是Ser531Leu,占58.33%(49/84),3株未检测出rpoB突变,基因型同表型的符合率为96.43%(81/84)。 结论 浙江地区MDR-TB流行株基因突变仍然集中在常见的基因位点,表明在本地区利用Xpert MTB/RIF筛查MDR-TB患者仍具有较高的应用价值。 Abstract:Objective To analyze the characteristics of genetic mutations associated with isoniazid (INH) and rifampicin (RIF)-resistant Mycobacterium tuberculosis (MDR-TB) isolates. Methods Culture positive sputum specimens from suspicious TB were subjected to species identification. Genomic DNAs of Mycobacterium tuberculosis complex (MTBC) were extracted. InhA, katG, and Rifampicin Resistance Determining Region (RRDR) of rpoB gene were amplified. Amplicons were performed with Sanger sequencing. Results A total of 3 317 TB were identified, including3 009 new treated, 308 retreated, and 84 MDR-TB, accounting for 2.53% (84/3 317). In INH resistant isolates, 47 had single-point mutation in katG (315 site) or inhA (-15 or -8 site), and 19 had both katG and inhA mutations. The coincidence rate of genotype with phenotype was 78.57% (66/84). The most frequent mutation was Ser315Thr (47.62%, 40/84). In RIF resistant isolates, 79 had mutations in rpoB (sites 511, 512, 513, 516, 522, 526 and 531), and 2 had mutations in 511/513 or 511/516. The most frequent mutation was Ser531Leu (58.33%, 49/84). The coincidence rate of genotype with phenotype was 96.43% (81/84). Conclusions Genetic mutations of inhA, katG and rpoB gene in Zhejiang are concentrated relatively on common gene sites, and Xpert MTB/RIF is still of high application value to screen MDR-TB in Zhejiang. -
Key words:
- Zhejiang /
- Tuberculosis /
- katG /
- rpoB /
- inhA /
- Genetic mutations
-
表 1 84例耐多药患者特征分析
Table 1. Analysis for characteristics of 84 MDR-TB patients
特征 例数 占比(%) 性别 男 56 66.67 女 28 33.33 年龄(岁) ≤30 25 29.76 30~ 21 25.00 ≥50 38 45.24 户籍 本地 61 72.62 外地 23 27.38 职业 体力劳动者 55 65.48 非体力劳动者 29 34.52 实验室检测 抗酸染色阳性 57 67.86 治疗结果 治愈或完成疗程 65 77.38 治疗中 11 13.10 失败 8 9.52 肺结核分类 原发性肺结核 12 14.29 继发性肺结核 72 85.71 表 2 84株表型耐药及10株表型敏感MTBC katG和inhA区突变位点分布情况
Table 2. Distributions of genetic mutations of katG and inhA among 84 INHR and 10 INHSa
株型 突变类型 位点 核苷酸和(或)氨基酸改变 株数(N=94) 核苷酸改变 氨基酸改变 耐药株 未检测出 无 无 无 18 单位点突变 katG 315 AGC→ACC Ser→Thr 21 AGC→TGC Ser→Cys 8 AGC→AAC Ser→Asn 5 inhA -15 C→T 无 9 inhA -8 T→C 无 4 双位点突变 katG 315/inhA -15 AGC→ACC Ser→Thr 13 C→T 无 katG 315/inhA -8 AGC→ACC Ser→Thr 6 T→C 无 敏感株 未检出 无 无 无 10 注:aINHR表示异烟肼耐药株,INHS表示异烟肼敏感株。 表 3 84株表型耐药及10株表型敏感MTBC rpoB核心区突变位点分布情况
Table 3. Distributions of genetic mutations of rpoB among 84 RIFR and 10 RIFSa
株型 突变类型 位点 核苷酸和(或)氨基酸改变 株数(N=94) 核苷酸改变 氨基酸改变 耐药株 未检测出 无 无 无 3 单位点突变 511 CTG→CCG Leu→Pro 1 512 AGC→ACC Ser→Thr 2 513 CAA→AAA Gln→Lys 1 516 GAC→GTC Asp→Val 3 522 TCG→TTG Ser→Leu 2 526 CAC→GAC His→Asp 13 CAC→CCC His→Pro 2 CAC→CTC His→Leu 1 CAC→CGC His→Arg 2 CAC→AAC His→Asn 1 531 TCG→TTG Ser→Leu 49 TCG→TGG Ser→Trp 2 双位点突变 511/513 CTG→CCG Leu→Pro 1 CAA→AAA Gln→Lys 511/516 CTG→CCG Leu→Pro 1 GAC→GTC Asp→Val 敏感株 未检出 无 无 无 10 注:aINHR表示异烟肼耐药株,INHS表示异烟肼敏感株。 -
[1] World Health Organization. Global tuberculosis report 2019 [EB/OL]. (2019-10-15) [2019-12-20]. https://www.who.int/publications-detail/global-tuberculosis-report-2019. [2] Zhao Y, Xu S, Wang L, et al. National survey of drug-resistant tuberculosis in China [J]. N Engl J Med, 2012, 366(23):2161-2170. DOI: 10.1056/nejmoa1108789. [3] Falzon D, Mirzayev F, Wares F, et al. Multidrug-resistant tuberculosis around the world: what progress has been made [J]. Eur Respir J, 2015, 45(1):150-160. DOI: 10.1183/09031936.00101814. [4] Zenteno-Cuevas R, Cuevas-Cordoba B, Parissi-Crivelli A. RpoB, katG and inhA mutations in multi-drug resistant strains of Mycobacterium tuberculosis clinical isolates from southeast Mexico [J]. Enferm Infecc Microbiol Clin, 2019, 37(5):307-313. DOI: 10.1016/j.eimc.2018.09.002. [5] Alvarez-Uria G, Reddy R. Differences in rpoB, katG and inhA mutations between new and previously treated tuberculosis cases using the GenoType MTBDRplus assay [J]. Infect Genet Evol, 2018, 59:48-50. DOI: 10.1016/j.meegid.2018.01.022. [6] Isakova J, Sovkhozova N, Vinnikov D, et al. Mutations of rpoB, katG, inhA and ahp genes in rifampicin and isoniazid-resistant Mycobacterium tuberculosis in Kyrgyz Republic [J]. BMC Microbiol, 2018, 18(1):22. DOI: 10.1186/s12866-018-1168-x. [7] Zhao LL, Huang MX, Xiao TY, et al. Prevalence, risk and genetic characteristics of drug-resistant tuberculosis in a tertiary care tuberculosis hospital in China [J]. Infect Drug Resist, 2019, 12:2457-2465. DOI: 10.2147/IDR.S209971. [8] Ramaswamy S, Musser JM. Molecular genetic basis of antimicrobial agent resistance in Mycobacterium tuberculosis: 1998 update [J]. Tuber Lung Dis, 1998, 79(1):3-29. DOI: 10.1054/tuld.1998.0002. [9] Jin DJ, Gross CA. Mapping and sequencing of mutations in the Escherichia coli rpoB gene that lead to rifampicin resistance [J]. J Mol Biol, 1988, 202(1):45-58. DOI: 10.1016/0022-2836(88)90517-7. [10] Prim RI, Schorner MA, Senna SG, et al. Molecular profiling of drug resistant isolates of Mycobacterium tuberculosis in the state of Santa Catarina, southern Brazil [J]. Mem Inst Oswaldo Cruz, 2015, 110(5):618-623. DOI: 10.1590/0074-02760150100. [11] 赵雁林, 逄宇.结核病实验室检验规程[M].北京: 人民卫生出版社, 2015: 1-260.Zhao YL, Peng Y. Laboratory test procedures for tuberculosis [M]. People's Medical Publishing House, 2015: 1-260. [12] Deggim-Messmer V, Bloemberg GV, Ritter C, et al. Diagnostic molecular Mycobacteriology in regions with low tuberculosis endemicity: combining real-time PCR assays for detection of multiple Mycobacterial pathogens with line probe assays for identification of resistance mutations [J]. EBioMedicine, 2016, 9:228-237. DOI: 10.1016/j.ebiom.2016.06.016. [13] Peng Y, Chen SH, Zhang L, et al. Multidrug-resistant tuberculosis burden among the new tuberculosis patients in Zhejiang Province: an observational study, 2009-2013 [J]. Chin Med J (Engl), 2017, 130(17):2021-2026. DOI: 10.4103/0366-6999.213413. [14] 张靓, 董恒进, 郜琳, 等.居民就诊意愿与基层医疗服务能力研究——以浙江兰溪为例[J].卫生经济研究, 2016, (8):19-22. DOI: 10.14055/j.cnki.33-1056/f.20160802.013.Zhang L, Dong HJ, Gao L, et al. Studies on residents' willingness to seek care and the ability of primary medical service capacity: Take Lanxi in Zhejiang Province as an example [J]. Health Economics Research, 2016, (8):19-22. DOI: 10.14055/j.cnki.33-1056/f.20160802.013. [15] Seifert M, Catanzaro D, Catanzaro A, et al. Genetic mutations associated with isoniazid resistance in Mycobacterium tuberculosis: a systematic review [J]. PLoS One, 2015, 10(3):e0119628. DOI: 10.1371/journal.pone.0119628. [16] Borrell S, Gagneux S. Infectiousness, reproductive fitness and evolution of drug-resistant Mycobacterium tuberculosis [J]. Int J Tuberc Lung Dis, 2009, 13(12):1456-1466. [17] Cohen T, Sommers B, Murray M. The effect of drug resistance on the fitness of Mycobacterium tuberculosis [J]. Lancet Infect Dis, 2003, 3(1):13-21. DOI: 10.1016/s1473-3099(03)00483-3. [18] Huyen MN, Cobelens FG, Buu TN, et al. Epidemiology of isoniazid resistance mutations and their effect on tuberculosis treatment outcomes [J]. Antimicrob Agents Chemother, 2013, 57(8):3620-3626. DOI: 10.1128/AAC.00077-13. [19] Luo T, Zhao M, Li X, et al. Selection of mutations to detect multidrug-resistant Mycobacterium tuberculosis strains in Shanghai, China [J]. Antimicrob Agents Chemother, 2010, 54(3):1075-1081. DOI: 10.1128/AAC.00964-09. [20] Zhao LL, Chen Y, Chen ZN, et al. Prevalence and molecular characteristics of drug-resistant Mycobacterium tuberculosis in Hunan, China [J]. Antimicrob Agents Chemother, 2014, 58(6):3475-3480. DOI: 10.1128/AAC.02426-14. [21] Luo D, Chen Q, Xiong G, et al. Prevalence and molecular characterization of multidrug-resistant M. tuberculosis in Jiangxi province, China [J]. Sci Rep, 2019, 9(1):7315. DOI: 10.1038/s41598-019-43547-2. [22] Kigozi E, Kasule GW, Musisi K, et al. Prevalence and patterns of rifampicin and isoniazid resistance conferring mutations in Mycobacterium tuberculosis isolates from Uganda [J]. PLoS One, 2018, 13(5):e0198091. DOI: 10.1371/journal.pone.0198091. [23] Luna JF, Montero H, Sampieri CL, et al. Sequencing of the entire rpob gene and characterization of mutations in isolates of Mycobacterium tuberculosis circulating in an endemic tuberculosis setting [J]. J Glob Antimicrob Resist, 2019, 19:98-103. DOI: 10.1016/j.jgar.2019.03.001. [24] Tavanaee SA, Ashna H, Kaffash A, et al. Mutations of rpob gene associated with rifampin resistance among Mycobacterium tuberculosis isolated in tuberculosis regional reference laboratory in northeast of Iran during 2015-2016 [J]. Ethiop J Health Sci, 2018, 28(3):299-304. DOI: 10.4314/ejhs.v28i3.7. [25] Koch AV, Mizrahi, Warner DF. The impact of drug resistance on Mycobacterium tuberculosis physiology: what can we learn from rifampicin [J]. Emerg Microbes Infect, 2014, 3(3):e17. DOI: 10.1038/emi.2014.17. -





 下载:
下载: