Association of leukocyte telomere length in peripheral blood and the risk of mild cognitive impairment in elderly subjects:a case-control study
-
摘要:
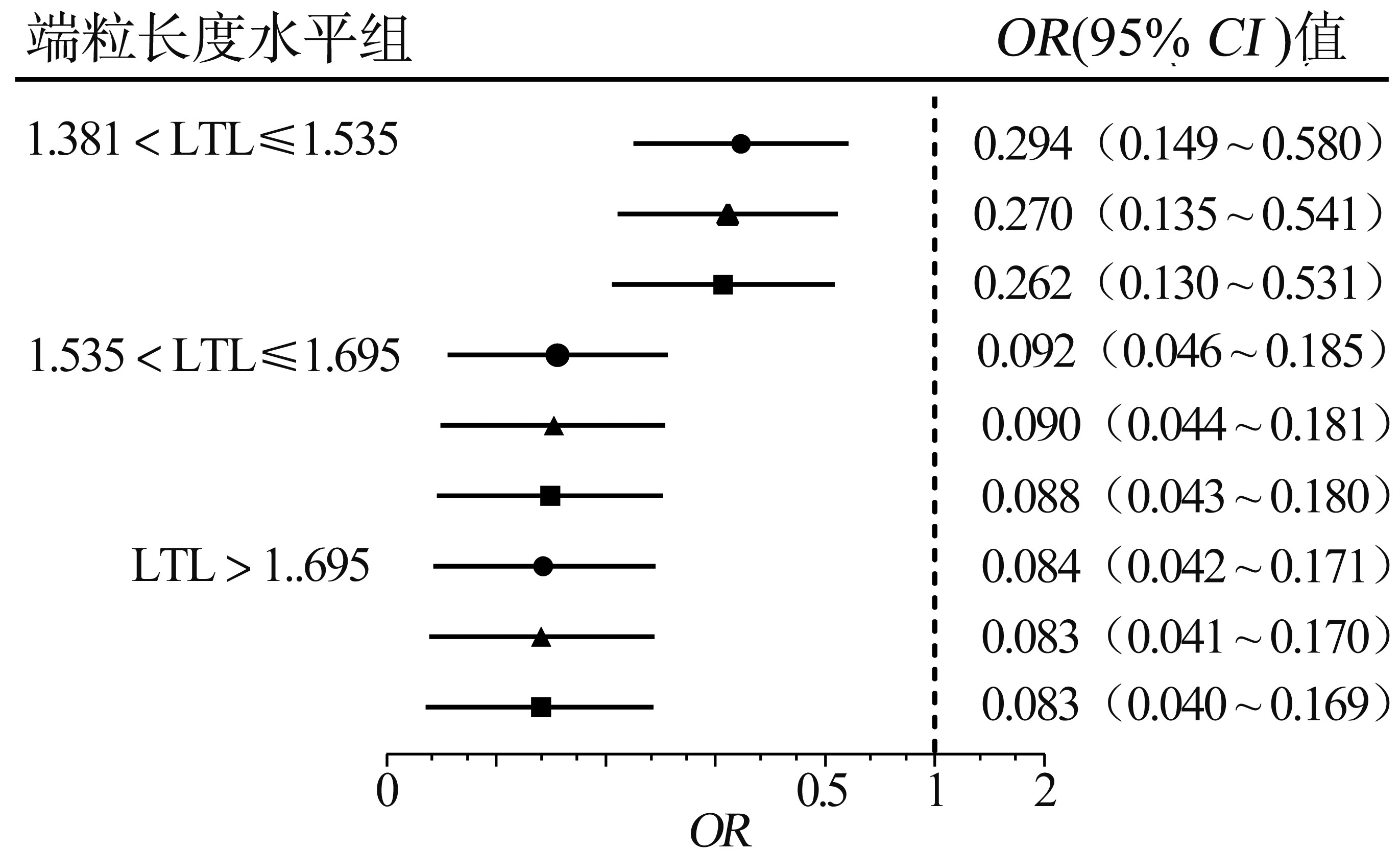
目的 探讨轻度认知障碍(mild cognitive impairment, MCI)老年人外周血白细胞端粒长度(leukocyte telomere length, LTL)与MCI发生风险之间的关联。 方法 于2017年10月-2017年12月在天津市南开区王顶堤街道的5个社区纳入MCI和健康对照老年受试者进行病例对照设计, 实施问卷调查和静脉血样采集, 用实时荧光定量多聚核苷酸链式反应(real-time quantitative polymerase chain reaction, qPCR)进行LTL的测量。采用多元Logistic回归分析模型分析LTL不同水平与MCI发生风险之间的关联。 结果 两组受试对象都为183例。MCI组LTL水平(1.44±0.23)低于对照组(1.66±0.23), 两组差异具有统计学意义(Z=-8.94, P < 0.001)。调整年龄、性别、教育程度、吸烟、饮酒、糖尿病、高血压、心脏病后, 较低水平LTL与MCI发生风险存在统计学意义关联(均有P < 0.05);与LTL第一水平组相比, 其二、三、四水平组MCI发生风险的比值比(odds ratio, OR)及95%置信区间(95% confidence interval, 95% CI)分别为0.262(0.130~0.531)、0.088(0.043~0.180)、0.083(0.040~0.169)。 结论 外周血白细胞短端粒可能是MCI发生的独立危险因素, 并且随着LTL缩短, MCI发生风险增加。 -
关键词:
- 轻度认知障碍 /
- 端粒 /
- 病例对照研究 /
- 多元Logistic回归分析模型
Abstract:Objective To explore the relationship between leukocyte telomere length(LTL)in peripheral blood and the risk of mild cognitive impairment(MCI)in elderly subjects. Methods This was a case-control study. Healthy controls and subjects with MCI were recruited from five communities in Wangdingdi Street, Nankai District, Tianjin from October 2017 to December 2017. Questionnaires were conducted and venous blood samples were collected from each subject. LTL was assessed using real-time quantitative polymerase chain reaction assay(qPCR). Multiple logistic regression model was performed to analyze the relationship between LTL for each level group and the risk of MCI. Results A total of 183 participants was enrolled for each group. The level of serum LTL was 1.44±0.23 in the MCI group, which was lower than 1.66±0.23 in the control group(Z=-8.94, P < 0.001). There was a significant correlation between lower levels of LTL and the risk for MCI after adjusting variables for age, gender, education, smoking, alcohol consumption, diabetes, hypertension, and heart disease(allP < 0.05). Compared with the first level group of LTL, the adjusted odds ratio(OR) and 95% confidence interval(95% CI) for the risk of MCI were 0.262(0.130-0.531) for the second level group, 0.088(0.043-0.180) for the third level group, and 0.083(0.040-0.169) for the fourth level group. Conclusions Short leukocyte telomere length in peripheral blood appears to be an independent risk factor for the incidence of MCI. And with the decrease of LTL, the risk of MCI increased. -
Key words:
- Mild cognitive impairment /
- Telomere /
- Case-control study /
- Multiple logistic regression
-
表 1 研究人群的人口统计学和临床特征[n(%)]
Table 1. Demographic and clinical characteristics of the study population[n(%)]
变量 MCI组(n=183) 对照组(n=183) t/Z/χ2值 P值 人口学变量 年龄(岁) 66.91±5.67 68.07±5.89 -2.07 0.039 男性 82(44.81) 64(34.97) 3.69 0.055 教育程度(年) 9.60±2.69 9.60±2.69 0.00 1.000 生活方式 BMI(kg/m2) 25.16±3.20 24.19±3.14 3.25 0.001 吸烟 13(7.10) 11(6.01) 0.18 0.673 饮酒 17(9.29) 16(8.74) 0.03 0.855 既往史 糖尿病 27(14.75) 26(14.21) 0.02 0.882 高血压 67(36.61) 71(38.80) 0.19 0.666 心脏病 11(6.01) 12(6.56) 0.05 0.830 生化指标 LTLa 1.44±0.23 1.66±0.23 -8.94 < 0.001 MMSEb 22.58±1.96 25.58±1.96 -12.63 < 0.001 注:aLTL表示为T/S比率-端粒DNA(T)与单拷贝基因(S)的拷贝数; bMMSE表示量表评分分值。 表 2 端粒长度与MCI发生风险关联的多元Logistic回归分析模型
Table 2. Multiple Logistic regression model analysis of relationship between telomere length and risk of MCI
端粒长度四分位数组别 例数 模型1a 模型2b 模型3c MCI组 对照组 OR(95% CI)值 P值 OR(95% CI)值 P值 OR(95% CI)值 P值 LTL≤1.381 76 16 1.000 1.000 1.000 1.381 < LTL≤1.535 53 38 0.294(0.149~0.580) 0.123 0.270(0.135~0.541) 0.215 0.262(0.130~0.531) 0.025 1.535 < LTL≤1.695 28 64 0.092(0.046~0.185) < 0.001 0.090(0.044~0.182) < 0.001 0.088(0.043~0.180) < 0.001 LTL > 1.695 26 65 0.084(0.042~0.171) < 0.001 0.083(0.041~0.170) < 0.001 0.083(0.040~0.169) < 0.001 注:a模型1未进行调整; b模型2调整年龄、性别、教育程度; c模型3调整年龄、性别、教育程度、吸烟、饮酒、糖尿病、高血压、心脏病。 -
[1] Alzheimer's Association. 2016 Alzheimer's disease facts and figures[J]. Alzheimers Dement, 2016, 12(4): 459-509. DOI: 10.1016/j.jalz.2016.03.001. [2] Wimo A. The end of the beginning of the Alzheimer's disease nightmare: a devil's advocate's view[J]. J Alzheimers Dis, 2018, 64(s1): S41-S46. DOI: 10.3233/JAD-179905. [3] Mancioppi G, Fiorini L, Timpano SM, et al. Novel technological solutions for assessment, treatment, and assistance in mild cognitive impairment[J]. Front Neuroinform, 2019, 13(58): 1-23. DOI: 10.3389/fninf.2019.00058. [4] Tedone E, Arosio B, Colombo F, et al. Leukocyte telomere length in Alzheimer's disease patients with a different rate of progression[J]. J Alzheimers Dis, 2015, 46(3): 761-769. DOI: 10.3233/JAD-142808. [5] Zhan YQ, Song C, Robert K, et al. Telomere length shortening and Alzheimer disease-a mendelian randomization study[J]. JAMA Neuro, 2015, 72(10): 1202-1203. DOI: 10.1001/jamaneurol.2015.1513. [6] Ma F, Lv X, Du Y, et al. Association of leukocyte telomere length with mild cognitive impairment and Alzheimer's disease: role of folate and homocysteine[J]. Dement Geriatr Cogn Disord, 2019, 1-12. DOI: 10.1159/000501958. [7] Scarabino D, Broggio E, Gambina G, et al. Leukocyte telomere length in mild cognitive impairment and Alzheimer's disease patients[J]. Exp Gerontol, 2017, 98: 143-147. DOI: 10.1016/j.exger.2017.08.025. [8] Lau H, Mat AF, Rajab NF, et al. Identification of neuroprotective factors associated with successful ageing and risk of cognitive impairment among Malaysia older adults[J]. Curr Gerontol Geriatr Res, 2017, 2017(7): 1-7. DOI: 10.1155/2017/4218756. [9] Roberts RO, Boardman LA, Cha RH, et al. Short and long telomeres increase risk of amnestic mild cognitive impairment[J]. Mech Ageing Dev, 2014, 1(141-142): 64-69. DOI: 10.1016/j.mad.2014.10.002. [10] Leigh H. Diagnostic and statistical manual of mental disorders(DSM)[M]. Encyclopedia of clinical neuropsychology cham; Springer International Publishing, 2018: 1-4. DOI: 10.1007/978-3-319-56782-2_9129-1. [11] 韩睿婕.病例对照分析法样本容量及基因外显率参数的估计方法[D].杨凌: 西北农林科技大学, 2009.Han RJ. Estimating sample size and penetrance parameters for case-control design of candidate-gene associations[D]. Yangling: Northwest A & F University, 2009. [12] Cawthon RM. Telomere measurement by quantitative PCR[J]. Nucleic acids research, 2002, 30(10): e47. DOI: 10.1093/nar/30.10.e47. [13] Innes KE, Selfe TK, Brundage K, et al. Effects of meditation and music-listening on blood biomarkers of cellular aging and Alzheimer's disease in adults with subjective cognitive decline: an exploratory randomized clinical trial[J]. J Alzheimers Dis, 2018, 66(3): 947-970. DOI: 10.3233/JAD-180164. [14] Sindi S, Ngandu T, Hovatta I, et al. Baseline telomere length and effects of a multidomain lifestyle intervention on cognition: the FINGER randomized controlled trial[J]. J Alzheimers Dis, 2017, 59(4): 1459-1470. DOI: 10.3233/JAD-170123. [15] Tonnies E, Trushina E. Oxidative stress, synaptic dysfunction, and Alzheimer's disease[J]. J Alzheimers Dis, 2017, 57(4): 1105-1121. DOI: 10.3233/JAD-161088. [16] Mota SI, Costa RO, Ferreira IL, et al. Oxidative stress involving changes in Nrf2 and ER stress in early stages of Alzheimer's disease[J]. Biochim Biophys Acta, 2015, 1852(7): 1428-1441. DOI: 10.1016/j.bbadis.2015.03.015. [17] Ahmed W, Lingner J. Impact of oxidative stress on telomere biology[J]. Differentiation, 2018, 99(21-27). DOI: 10.1016/j.diff.2017.12.002. [18] Cai Z, Yan LJ, Ratka A. Telomere shortening and Alzheimer's disease[J]. Neuromolecular Med, 2013, 15(1): 25-48. DOI: 10.1007/s12017-012-8207-9. -





 下载:
下载: