Molecular characteristic of the two surface glycoproteins of highly pathogenic avian influenza A H5N6 viruses from human, 2014-2018, China
-
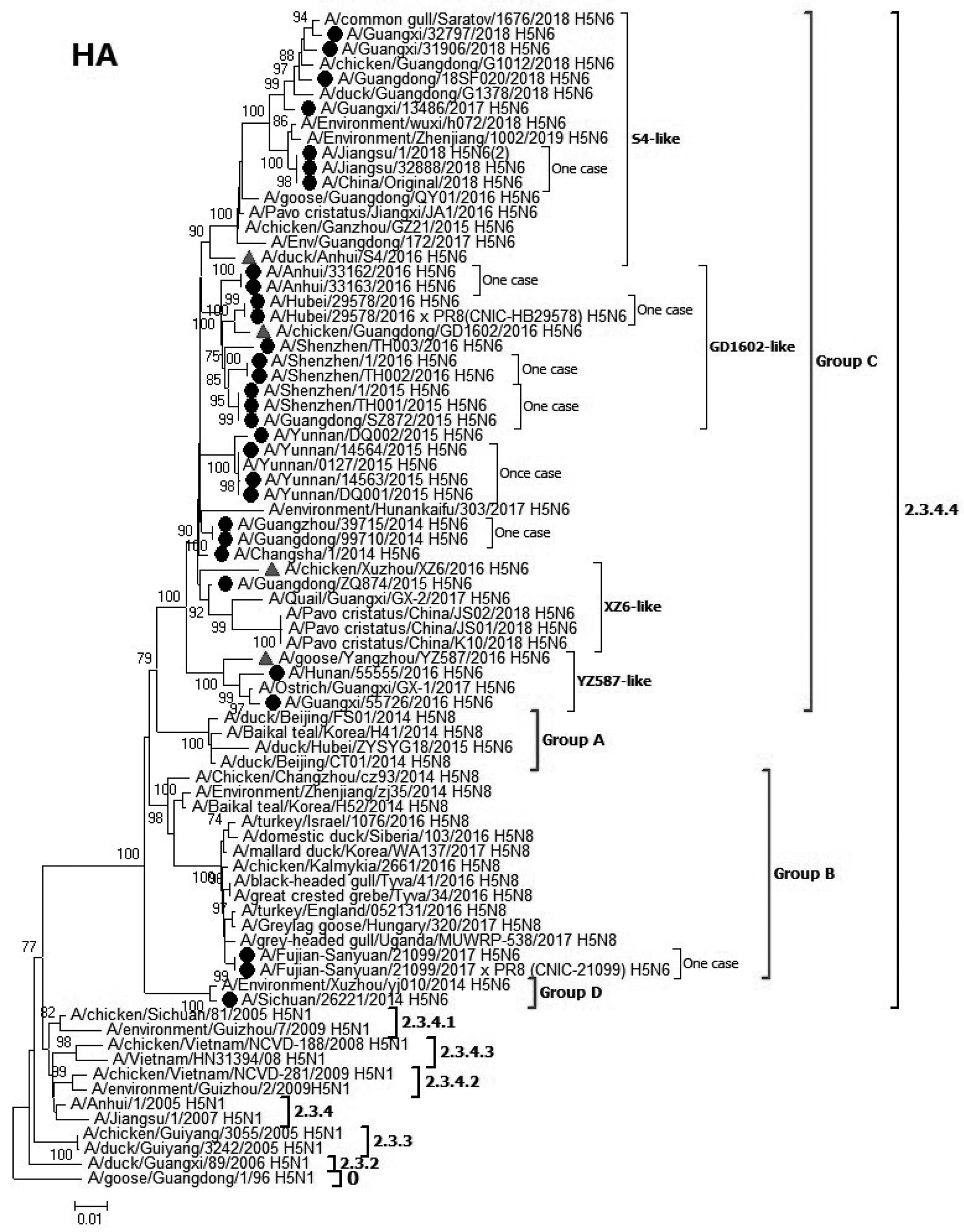
摘要:
目的 对19例人感染高致病性H5N6禽流感病毒的血凝素(hemagglutinin, HA)和神经氨酸酶(neuraminidase, NA)蛋白进行分子进化分析。 方法 运用下一代测序平台对病毒分离物进行全基因组测序, 从美国国家生物技术信息中心(national center for biotechnology information, NCBI)和全球流感序列数据库(global initiative on sharing avian influenza data, GISAID)下载参考序列, 利用Blasts、Mega 6.1及Clustal X 2.1等软件进行序列分析。 结果 2014-2018年中国共发生23例人感染H5N6禽流感病毒病例。对19个病例的H5N6病毒的HA和NA基因进行进化分析。HA进化分析显示病毒都属于Clade 2.3.4.4, 其中涉及17个病例的病毒属于Group C; 首例H5N6病例毒株(A/Sichuan/26221/2014)属于Group D; 福建一个病例(A/Fujian-Sanyuan/21099/2017)属于Group B。所有19个病例的病毒HA蛋白的裂解位点含有多个碱性氨基酸。所有病毒的HA蛋白的受体结合位点226~228位氨基酸是QS(R)G(氨基酸排序以H3-HA为准), 理论上对禽类受体α2-3半乳糖苷唾液酸(SAα2-3Gal)有嗜性。18病例病毒的HA蛋白发生了T160A的突变, 导致在158N位点失去糖基化。除了A/Sichuan/26221/2014外, 18个病例的病毒NA蛋白在58~68位缺失了10个氨基酸。9个病例的病毒PB2蛋白发生E627K突变。 结论 2014-2018年间中国人感染H5N6病毒进化活跃, 具有明显的基因多样性, 需要加强对病毒分子进化的监测。 Abstract:Objective To analyze the molecular characteristic of hemagglutinin(HA) and neuraminidase(NA)proteins of highly pathogenic avian influenza H5 N6 viruses from 19 human cases. Methods Whole-genome sequences of original specimen and virus isolates were obtained by next-generation sequencing technology. Reference sequence information was collected from national center for biotechnology information(NCBI) and global initiative on sharing avian influenza data(GISAID) database. Pairwise sequence alignments and phylogenetic analysis were performed by Blasts, Clustal X 2.1 and Mega 6.1 softwares. Results A total of 23 cases of human infection with H5 N6 avian influenza virus occurred in China from 2014 to 2018. The HA and NA genes of H5 N6 virus from 19 cases were analyzed. HA evolutionary analysis showed that viruses belonged to Clade 2.3.4.4, of which 17 cases belonged to Group C; the first H5 N6 strain(A/Sichuan/26221/2014) belonged to Group D; and a Fujian case strain(A/Fujian-Sanyuan/21099/2017) belonged to Group B. The cleavage sites of HA protein of 29 strains of viruses in all 19 cases contained multiple basic amino acids. The 226-228 amino acids at the receptor binding sites of HA proteins of all viruses were QS(R) G(amino acids are sequenced according to the HA sequence of H3 subtype). In addition to one case in Guangzhou in 2014, the HA protein of 18 isolates mutated in T160 A, resulting in loss of glycosylation at 158 N site. In addition to A/Sichuan/26221/2014, 10 amino acids were deleted in 58-68 sites of NA proteins of strains from 18 cases. E627 K mutation occurred in 17 strains of virus PB2 protein from 9 cases. Conclusions From 2014 to 2018, the evolution of H5 N6 virus infection in China is active, and there is obvious genetic diversity. It is necessary to strengthen the monitoring of the evolution of H5 N6 viruses in China. -
Key words:
- Avian influenza virus /
- H5N6 subtype /
- Human cases /
- Molecular evolution
-
表 1 2014-2018年31株人感染H5N6病毒编码蛋白关键氨基酸位点分析
Table 1. Key amino acid mutations of the 31 H5N6 viruses form human, 2014-2018
蛋白 生物学功能 突变 31株H5N6人病毒 A/Jiangsu/1/2018 A/Sichuan/26221/2014 A/Hubei/29578/2016 A/Fujian-Sanyuan/21099/2017 HA 受体结合位点(H3排序) Q226L Q(31) Q Q Q Q S/R227N S(10), R(17), G(3), H(1) R R S R G228S G(31) G G G G 丧失158位糖基化位点 T160A A(27), T(2), S(2) A A A A 获得124位糖基化位点 126缺失 缺失(23), E(8) 缺失 E 缺失 E 裂解位点 REKRRK↓G(2), RERRRK↓G REKRRK↓G RERRRK↓G RERRRK↓G REKRRK↓G RERRRK↓G(25), RERRRK↓G(25), REKRRKKR↓G(2), RETR↓G(2) NA 茎区 58-68缺失 是(30), 否(1) 是 否 是 是 H274Y H H H H H R371K R R R R R PB2 增强对小鼠毒力 Q591K Q (31) Q Q Q Q 哺乳动物的适应性 E627K E (13), K(17), X(1) E E E E 增强对小鼠毒力 D701N D(30), N(1) D N D D PB1 增强对雪貂毒力 I368V I(17), V(14) I I V I PB1-F2 增强哺乳动物毒力 87-90氨基酸 90(14), 57(1) 52氨基酸 57氨基酸 52氨基酸 52氨基酸 PA 宿主信号 V100A V(21), A(9), I(1) V V V I S409N S(15), N(16) S S N S M2 病毒抗性 S31N S(14), N(17) S S N N NS1 改变对小鼠毒力 80-84缺失 Yes(14), No(17) Yes Yes No No C-末端PED基序 227-300氨基酸 ESEV(14), GSEV(2), ESEV ESEV 截短缺失 GSEV RSEV(2), 截短缺失(13) 增强对小鼠毒力 D92E E(14), D(17) E E D D P42S S (31) S S S S -
[1] Tong S, Zhu X, Li Y, et al. New world bats harbor diverse influenza A viruses[J]. PLoS Pathog, 2013, 9(10): e1003657. DOI: 10.1371/journal.ppat.1003657. [2] Fouchier RA, Munster V, Wallensten A, et al. Characterization of a novel influenza A virus hemagglutinin subtype(H16)obtained from black-headed gulls[J]. J Virol, 2005, 79(5): 2814-2822. DOI: 10.1128/JVI.79.5.2814-2822.2005. [3] Lee DH, Bertran K, Kwon JH, et al. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3. 4.4[J]. J Vet Sci, 2017, 18(S1): 269-280. DOI: 10.4142/jvs.2017.18.S1.269. [4] WHO/OIE/FAO H5N1 Evolution Working Group. Toward a unified nomenclature system for highly pathogenic avian influenza virus(H5N1)[J]. Emerg Infect Dis, 2008, 14(7): e1. DOI: 10.3201/eid1407.071681. [5] Qi X, Cui L, Yu H, et al. Whole-genome sequence of a reassortant H5N6 avian influenza virus isolated from a live poultry market in China, 2013[J]. Genome Announc, 2014, 2(5): e00706-14. DOI: 10.1128/genomeA.00706-14. [6] Bi Y, Chen Q, Wang Q, et al. Genesis, evolution and prevalence of H5N6 avian influenza viruses in China[J]. Cell Host Microbe, 2016, 20(6): 810-821. DOI: 10.1016/j.chom.2016.10.022. [7] Sun W, Li J, Hu J, et al. Genetic analysis and biological characteristics of different internal gene origin H5N6 reassortment avian influenza virus in China in 2016[J]. Vet Microbiol, 2018, 219: 200-211. DOI: 10.1016/j.vetmic.2018.04.023. [8] Jiang H, Lai SJ, Qin Y, et al. A review of global human infection with avian influenza and epidemiological characteristics[J]. Chinese Science Bulletin. 2017, 62(19): 2104-2115. DOI: 10.1360/N972017-00267. [9] 邓斐, 彭杰夫, 崔仑标, 等. 1例人感染H7N4禽流感病毒分子溯源研究[J].中华微生物学和免疫学杂志, 2018, 38(9): 665-672. DOI: 10.3760/cma.j.issn.0254-5101.2018.09.004.Deng F, Peng JF, Cui LB, et al. Genetic origin of avian influenza A H7N4 virus causing a case of human infection in China[J]. Chin J Microbiol Immunol. 2018, 38(9): 665-672. DOI: 10.3760/cma.j.issn.0254-5101.2018.09.004. [10] Hoffmann E, Stech J, Guan Y, et al. Universal primer set for the full-length amplification of all influenza A viruses[J]. Arch Virol, 2001, 146(12): 2275-2289. DOI: 10.1007/s007050170002. [11] Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees[J]. BMC Evol Biol, 2007, 7: 214. DOI: 10.1186/1471-2148-7-214. [12] Global influenza information network. China: CHP notified of human case of avian influenza A(H5N6)in Jiangsu[EB/OL]. (2018-11-23)[2019-12-06]. http://www.flu.org.cn/en/news-19950.html. [13] Lee DH, Bahl J, Torchetti MK, et al. Highly pathogenic avian influenza viruses and generation of novel reassortants, United States, 2014-2015[J]. Emerg Infect Dis. 2016, 22(7): 1283-1285. DOI: 10.3201/eid2207.160048. [14] Herfst S, Schrauwen EJ, Linster M, et al. Airborne transmission of influenza A/H5N1 virus between ferrets[J]. Science, 2012, 336(6088): 1534-1541. DOI: 10.1126/science.1213362. [15] Russell CA, Fonville JM, Brown AE., et al. The potential for respiratory droplet-transmissible A/H5N1 influenza virus to evolve in a mammalian host[J]. Science, 2012, 336(6088): 1541-1547. DOI: 10.1126/science.1222526. [16] Zhang W, Shi Y, Lu X, et al. An airborne transmissible avian influenza H5 hemagglutinin seen at the atomic level[J]. Science, 2013, 340(6139): 1463-1467. DOI: 10.1126/science.1236787. [17] Li M, Liu H, Bi Y, et al. Highly pathogenic avian influenza A(H5N8)virus in wild migratory birds, Qinghai Lake, China[J]. Emerg Infect Dis, 2017, 23(4): 637-641. DOI: 10.3201/eid2304.161866. [18] Poen MJ, Venkatesh D, Bestebroer TM, et al. Co-circulation of genetically distinct highly pathogenic avian influenza A clade 2.3. 4.4(H5N6)viruses in wild waterfowl and poultry in Europe and East Asia, 2017-2018[J]. Virus Evol, 2019, 22, 5(1): vez004. DOI: 10.1093/ve/vez004. [19] Bi Y, Tan S, Yang Y, et al. Clinical and immunological characteristics of human infections with H5N6 avian influenza virus[J]. Clin Infect Dis, 2019, 68(7): 1100-1109. DOI: 10.1093/cid/ciy681. [20] Yang L, Zhu W, Li X, et al. Genesis and dissemination of highly pathogenic H5N6 avian influenza viruses[J]. J Virol, 2017, 91(5): e02199-16. DOI: 10.1128/JVI.02199-16. [21] Sun H, Pu J, Wei Y, et al. Highly pathogenic avian influenza H5N6 viruses exhibit enhanced affinity for human type sialic acid receptor and in-contact transmission in model ferrets[J]. J Virol, 2016, 90(14): 6235-6243. DOI: 10.1128/JVI.00127-16. [22] Herfst S, Mok CKP, van den Brand JMA, et al. Human Clade 2.3. 4.4 A/H5N6 Influenza Virus Lacks Mammalian Adaptation Markers and Does Not Transmit via the Airborne Route between Ferrets[J]. mSphere, 2018, 3(1): e00405-17. DOI: 10.1128/mSphere.00405-17. -





 下载:
下载: