Correlation between bisphenol A level and activation of NLRP3 inflammasome in adipose tissue of obese patients
-
摘要:
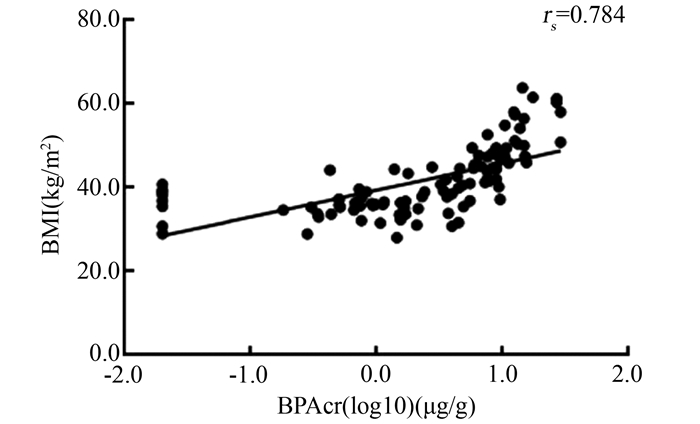
目的 通过临床病例样本检测分析探讨双酚A(bisphenol A, BPA)暴露与肥胖患者脂肪组织NLRP3炎症小体激活的相关性。 方法 纳入代谢减重手术的肥胖患者102例,收集临床资料及人口统计学数据,并采集血、尿和手术切除的内脏脂肪组织标本。液相色谱-质谱联法(liquid chromatography mass spectrometry, LC-MS)检测尿BPA浓度(肌酐校正BPA,简称BPAcr),ELISA检测血清、脂肪组织炎性细胞因子白细胞介素(interleukin, IL)-1β和IL-18含量,免疫荧光法检测脂肪组织NLRP3表达,qRT-PCR和免疫印迹法检测脂肪组织NLRP3激活相关分子的mRNA和蛋白表达水平,分析尿BPAcr与血清和脂肪组织炎性因子水平,以及脂肪组织NLRP3激活的相关性。 结果 肥胖患者尿BPAcr与BMI、血清IL-1β、血清IL-18、脂肪组织炎性因子IL-1β和IL-18水平呈正相关(rs值分别为0.784、0.852、0.737、0.509及0.471,均有P < 0.001)。肥胖患者脂肪组织巨噬细胞(adipose tissue macrophages, ATMs)中NLRP3表达,NLRP3、ASC mRNA表达水平,以及IL-1β、caspase-1蛋白表达水平与尿BPAcr均相关(均有P < 0.05)。 结论 BPA暴露可能通过激活脂肪组织ATMs中NLRP3炎症小体,上调促炎细胞因子的分泌,导致肥胖患者炎症状态。 Abstract:Objective The correlation between bisphenol A (BPA) exposure and the activation of NLRP3 inflammasomes in adipose tissue of obese patients was explored through the detection and analysis of clinical case samples. Methods A total of 102 obese patients undergoing metabolic surgery were included, and clinical data, demographic data, blood, urine, and surgically removed visceral fat tissue specimens were collected. Liquid chromatography mass spectrometry (LC-MS) was used to detect urine BPA concentration (Creatinine corrected BPA, BPAcr for short), and ELISA was used to detect serum and adipose tissue inflammatory cytokines IL-1β and IL-18 content. Immunofluorescence was used to detect the expression of NLRP3 in adipose tissue, qRT-PCR and immunoblot were used to detect the mRNA and protein expression levels of NLRP3 activation related molecules in adipose tissue. The correlation between urinary BPAcr and inflammatory factors in serum and adipose tissue, as well as the activation of NLRP3 in adipose tissue were analyzed. Results Urine BPAcr in obese patients was significantly positively correlated with body mass index (BMI), serum IL-1β, serum IL-18 adipose tissue inflammatory factors IL-1β and IL-18 levels (rs values were 0.784, 0.852, 0.737, 0.509, 0.471, all P < 0.001). The expression of NLRP3, the expression levels of NLRR3, ASC mRNA, and the protein expression levels of IL-1β and caspase-1 in ATMs of obese patients were significantly correlated with urinary BPAcr (all P < 0.05). Conclusion BPA exposure may activate the NLRR3 inflammasome in ATMs of adipose tissue and up-regulate the secretion of pro-inflammatory cytokines, leading to the inflammatory state of obese patients. -
Key words:
- Bisphenol A /
- Obesity /
- NLRR3 inflammasome /
- Inflammation /
- Adipose tissue
-
表 1 引物序列表
Table 1. Primer sequence listing
基因 上游引物 下游引物 IL-1β GCGGCATCCAGCTACGAATCTC CGGAGCGTGCAGTTCAGTGATC IL-18 AAGATGGCTGCTGAACCAGT GAGGCCGATTTCCTTGGTCA Caspase-1 ATGGACAAGTCAAGCCGCACAC TCCCACAAATGCCTTCCCGAATAC NLRR3 GCCCAAGGAGGAAGAGGAGGAG GCTTCTGGTTGCTGCTGAGGAC ASC TGGACGCCTTGGACCTCACC GAGCATCCAGCAGCCACTCAAC β-actin TCGTGCGTGACATTAAGGAGAAGC GGCGTACAGGTCTTTGCGGATG 表 2 研究对象的一般特征[M(P25, P75)]
Table 2. Characteristics of the participants [M(P25, P75)]
变量 合计 尿BPACr水平的四分位数a P值 1st 2nd 3rd 4th 人数[n(%)] 102(100.0) 25(24.5) 26(25.5) 26(25.5) 25(24.5) 年龄(x±s, 岁) 31.44±8.07 35.32±9.61 33.04±7.66 27.08±4.46 30.44±7.74 0.001 性别[n(%)] 0.053 男 47(46.1) 10(21.3) 7(14.9) 16(34.0) 14(29.7) 女 55(53.9) 15(27.3) 19(34.5) 10(18.2) 11(20.0) BMI (x±s, kg/m2) 41.72±8.02 35.82±3.53 36.81±4.11 42.95±5.26 51.42±7.05 < 0.001 FBG(mmol/L) 5.68(4.90, 7.73) 5.82(4.87, 8.13) 5.56(4.84, 6.55) 5.32(4.85, 6.82) 6.10(5.44, 9.79) 0.134 SBP(mm Hg) 134.00(117.00, 150.50) 121.00(108.00, 146.00) 138.00(115.00, 147.50) 135.00(126.00, 152.25) 138.00(121.00, 160.00) 0.233 DBP(mm Hg) 85.00(74.00, 97.50) 78.00(73.50, 99.00) 87.00(73.50, 101.50) 85.50(76.00, 99.00) 84.00(73.50, 93.50) 0.710 TC(mmol/L) 4.77(4.06, 5.65) 4.82(4.43, 5.72) 4.78(4.10, 5.64) 4.59(3.84, 6.01) 4.93(3.94, 5.53) 0.933 TG (mmol/L) 1.82(1.28, 2.68) 1.82(1.18, 2.96) 2.46(1.33, 3.07) 1.56(1.27, 2.44) 1.80(1.27, 2.13) 0.537 LDL-C (mmol/L) 2.99(2.45, 3.68) 2.96(2.38, 3.43) 3.05(2.55, 3.58) 2.97(2.39, 3.92) 2.99(2.51, 3.71) 0.808 HDL-C c(mmol/L) 0.97(0.84, 1.11) 1.00(0.89, 1.26) 0.97(0.88, 1.08) 0.97(0.82, 1.09) 0.94(0.82, 1.12) 0.396 注:a尿BPAcr四分位数间距分组(1st,2nd,3rd,4th分别表示第1、2、3、4个四分位数)。 -
[1] Andujar N, Galvez-Ontiveros Y, Zafra-Gomez A, et al. Bisphenol a analogues in food and their hormonal and obesogenic effects: a review[J]. Nutrients, 2019, 11(9): 2136. DOI: 10.3390/nu11092136. [2] Di Ciaula A, Portincasa P. Diet and contaminants: driving the rise to obesity epidemics?[J]. Curr Med Chem, 2019, 26(19): 3471-3482. DOI: 10.2174/0929867324666170518095736. [3] Lakind JS, Goodman M, Mattison DR. Bisphenol A and indicators of obesity, glucose metabolism/type 2 diabetes and cardiovascular disease: a systematic review of epidemiologic research[J]. Crit Rev Toxicol, 2014, 44(2): 121-150. DOI: 10.3109/10408444.2013.860075. [4] Moon MK, Jeong IK, Jung Oh T, et al. Long-term oral exposure to bisphenol A induces glucose intolerance and insulin resistance[J]. J Endocrinol, 2015, 226(1): 35-42. DOI: 10.1530/JOE-14-0714. [5] 李艳茹, 吴亚, 冯月梅, 等. 反式脂肪酸与慢性非传染性疾病关系研究进展[J]. 中华疾病控制杂志, 2020, 24(11): 1332-1337. DOI: 10.16462/j.cnki.zhjbkz.2020.11.017.Li YR, Wu ya, Feng YM, et al. Research progress on the relationship between trans fatty acids and chronic non communicable diseases[J]. Chin J Dis Control Prev, 2020, 24(11): 1332-1337. DOI: 10.16462/j.cnki.zhjbkz.2020.11.017. [6] Engin A. The Pathogenesis of Obesity-Associated Adipose Tissue Inflammation[J]. Obesity & Lipotoxicity, 2017, 960: 221-245. DOI: 10.1007/978-3-319-48382-5. [7] Rheinheimer J, de Souza BM, Cardoso NS, et al. Current role of the NLRP3 inflammasome on obesity and insulin resistance: a systematic review[J]. Metab: Clin Exp, 2017, 74: 1-9. DOI: 10.1016/j.metabol.2017.06.002. [8] 李应配, 罗时猛, 冷银芝, 等. 双酚A诱导肥胖小鼠脂肪组织巨噬细胞聚集[J]. 安徽医科大学学报, 2016, 51(10): 1464-1467. DOI: 10.19405/j.cnki.issn1000-1492.2016.10.015.Li YP, Luo SM, Leng YZ, et al. Adipose tissue macrophage aggregation induced by bisphenol A in obese mice[J]. Acta Universitatis Medicinalis Anhui, 2016, 51(10): 1464-1467. DOI: 10.19405/j.cnki.issn1000-1492.2016.10.015. [9] Boutens L, Hooiveld GJ, Dhingra S, et al. Unique metabolic activation of adipose tissue macrophages in obesity promotes inflammatory responses[J]. Diabetologia, 2018, 61(4): 942-953. DOI: 10.1007/s00125-017-4526-6. [10] Li R, Yang S, Gao R, et al. Relationship between the environmental endocrine disruptor bisphenol a and dyslipidemia: a five-year prospective study[J]. Endocr Pract, 2020, 26(4): 399-406. DOI: 10.4158/ep-2019-0384. [11] Bellavia A, Cantonwine DE, Meeker JD, et al. Pregnancy urinary bisphenol-A concentrations and glucose levels across BMI categories[J]. Environ Int, 2018, 113: 35-41. DOI: 10.1016/j.envint.2018.01.012. [12] Stojanoska MM, Milosevic N, Milic N, et al. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism disorders[J]. Endocrine, 2017, 55(3): 666-681. DOI: 10.1007/s12020-016-1158-4. [13] Collin D, Takeishi K, Guzman-Lepe J, et al. Generation of human fatty livers using custom-engineered induced pluripotent stem cells with modifiable SIRT1 metabolism[J]. Cell Metab, 2019, 30(2): 385-401. DOI: 10.1016/j.cmet.2019.06.017. [14] Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders[J]. Nature, 2017, 542(7640): 177-185. DOI: 10.1038/nature21363. [15] Swanson KV, Deng M, Ting JP. The NLRP3 inflammasome: molecular activation and regulation to therapeutics[J]. Nat Rev Immunol, 2019, 19(8): 477-489. DOI: 10.1038/s41577-019-0165-0. [16] Kelley N, Jeltema D, Duan Y, et al. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation[J]. Int J Mol Sci, 2019, 20(13): 3328. DOI: 10.3390/ijms20133328. -





 下载:
下载: