Identification of immune molecular markers for ischemic stroke based on genomic data
-
摘要:
目的 基于基因组学数据,探索缺血性脑卒中免疫相关分子标志物,为缺血性脑卒中的预防和临床治疗提供理论依据。 方法 选取基因表达综合数据库(gene expression omnibus, GEO)中GSE16561和GSE58294两个数据,对缺血性脑卒中相关免疫分子标志物进行分析和探索。基于免疫学数据库和分析网站(the immunology database and analysis portal, ImmPort)数据库,获取免疫相关的基因,分析其在缺血性脑卒中组和正常对照组中的差异表达,鉴定差异表达免疫基因(differentially expressed immune genes, DEIGs),并利用蛋白质互作网络(protein-protein interaction, PPI)鉴定免疫相关的关键基因。基于DEIGs进行通路富集分析,发现缺血性脑卒中可能富集的分子信号通路。最后,基于CIBERSORT算法评估22种免疫细胞的浸润丰度,并计算其在缺血性脑卒中组和正常对照组中的差异,以此推断缺血性脑卒中相关的免疫细胞。 结果 差异分析结果表明37个DEIGs在两组间差异均有统计学意义(均P<0.05),包含缺血性脑卒中样本上调基因31个,下调基因6个。通路分析结果表明鉴定的DEIGs主要富集于免疫相关分子通路上。PPI的分析结果显示,TLR4、TLR2、MMP-9、CCR7、STAT3、TNFSF13B、S100A12、CD19、CAMP和SLC11A1是缺血性脑卒中密切相关的关键免疫基因。CIBERSORT结果表明,与正常对照样本相比较,缺血性脑卒中样本免疫回应细胞的富集水平较低(均P<0.05),而免疫抑制细胞的水平较高(均P < 0.05)。 结论 研究基于转录组表达数据鉴定了缺血性脑卒中相关的免疫基因、免疫信号通路和免疫细胞,从3个分子水平上揭示了与缺血性脑卒中相关的免疫分子标志物,对缺血性脑卒中的有效防控和治疗策略的制定具有重要意义。 Abstract:Objective To explore the immune-related molecular markers for ischemic stroke based on genomic data, and to provide theoretical basis for the prevention and clinical treatment of ischemic stroke. Methods GSE16561 and GSE58294 from the gene expression omnibus (GEO) database were used for the analysis and exploration of molecular markers immune-related to ischemic stroke. Based on the immunology database and analysis portal (ImmPort) database, immune-related genes were obtained and their differential expression in ischemic stroke group and normal controls group were also analyzed to identify differentially expressed immune genes (DEIGs). Immune-related hub genes were identified by protein-protein interaction (PPI). Pathway analysis of DEIGs was performed to find the possible molecular signaling pathways of ischemic stroke. Finally, the infiltration abundance of 22 immune cells was evaluated based on the CIBERSORT algorithm, and the difference between ischemic stroke group and normal control group was subsequently calculated to determine ischemic stroke-related immune cells. Results Differential analysis results showed that 37 DEIGs had statistically significant differences between two groups (all P < 0.05), including 31 up-regulated genes and 6 down-regulated genes in ischemic stroke samples. Pathway analysis results showed that the identified DEIGs were mainly enriched in immune-related molecular pathways. PPI analysis results showed that TLR4, TLR2, MMP-9, CCR7, STAT3, TNFSF13B, S100A12, CD19, CAMP and SLC11A1 were key hub immune genes closely related to ischemic stroke. Based on CIBERSORT results, we found that immune response cells were less enriched in ischemic stroke samples (all P < 0.05). However, the levels of immunosuppressive cells were higher (all P < 0.05). Conclusions Based on transcriptome expression data, this study identifies immune genes, immune signaling pathways and immune cells associated with ischemic stroke. This study reveals the immune molecular markers associated with ischemic stroke at three molecular levels, which is of great significance for the effective prevention and control of ischemic stroke and the formulation of targeted treatment strategies. -
Key words:
- Ischemic stroke /
- Immune genes /
- Signaling pathways /
- Immune cells /
- Molecular markers /
- Omics data
-
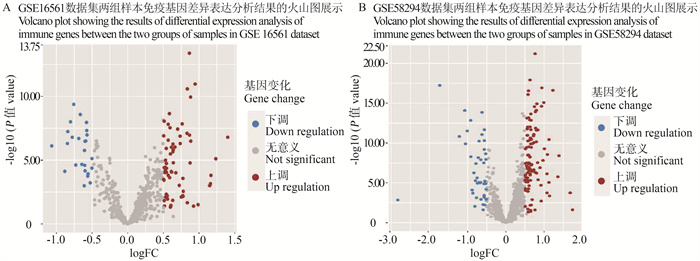
图 1 GSE16561和GSE58294数据集缺血性脑卒中和正常对照样本免疫基因差异表达分析结果的火山图
蓝色表示缺血性脑卒中样本表达下调基因;红色表示表达上调基因;FC:对数转换的倍数值。
Figure 1. Volcano plots results of differential expression analysis of immune genes in ischemic stroke and normal control samples from GSE16561 and GSE58294 datasets
Blue indicates down-regulated genes in ischemic stroke samples; Red indicates up-regulated genes; FC: fold change.
图 3 缺血性脑卒中相关DEIGs的通路富集分析结果
DEIGs:差异表达免疫基因;A:KEGG富集分析结果;B:GO富集分析结果;KEGG:京都基因和基因组百科全书;GO:基因本体;红色表示免疫相关信号通路。
Figure 3. Results of pathway enrichment analysis of DEIGs associated with ischemic stroke
DEIGs: differentially expressed immune genes; A: KEGG enrichment results; B: GO enrichment results; KEGG: Kyoto encyclopedia of genes and genomes; GO: gene ontology; Red indicates immune-related signaling pathways.
图 5 GSE16561和GSE58294数据集缺血性脑卒中组和正常对照组免疫细胞浸润分析结果
红色表示缺血性脑卒中样本浸润水平降低的免疫细胞,绿色表示缺血性脑卒中样本浸润水平升高的免疫细胞;a: P<0.05, b: P<0.01。
Figure 5. Analysis results of immune cell infiltration in ischemic stroke group and normal controls group in GSE16561 and GSE58294 datasets
Immune cells with reduced levels of infiltration in ischemic stroke samples are shown in red and those with increased levels in green. a: P < 0.05, b: P < 0.01.
-
[1] Collaborators G2S. Global, regional, and national burden of stroke, 1990-2016: a systematic analysis for the global burden of disease study 2016 [J]. Lancet Neurol, 2019, 18(5): 439-458. DOI: 10.1016/S1474-4422(19)30034-1. [2] Harston GWJ, Rane N, Shaya G, et al. Imaging biomarkers in acute ischemic stroke trials: a systematic review [J]. AJNR Am J Neuroradiol, 2015, 36(5): 839-843. DOI: 10.3174/ajnr.A4208. [3] Zameer S, Siddiqui AS, Riaz R. Multimodality imaging in acute ischemic stroke [J]. Curr Med Imag Former Curr Med Imag Rev, 2021, 17(5): 567-577. DOI: 10.2174/1573405616666201130094948. [4] Shi KB, Zou M, Jia DM, et al. tPA mobilizes immune cells that exacerbate hemorrhagic transformation in stroke [J]. Circ Res, 2021, 128(1): 62-75. DOI: 10.1161/CIRCRESAHA.120.317596. [5] Iadecola C, Buckwalter MS, Anrather J. Immune responses to stroke: mechanisms, modulation, and therapeutic potential [J]. J Clin Invest, 2020, 130(6): 2777-2788. DOI: 10.1172/JCI135530. [6] Xu SB, Lu JN, Shao AW, et al. Glial cells: role of the immune response in ischemic stroke [J]. Front Immunol, 2020, 11: 294. DOI: 10.3389/fimmu.2020.00294. [7] Nikolic D, Jankovic M, Petrovic B, et al. Genetic aspects of inflammation and immune response in stroke [J]. Int J Mol Sci, 2020, 21(19): 7409. DOI: 10.3390/ijms21197409. [8] Wu F, Liu ZC, Zhou LH, et al. Systemic immune responses after ischemic stroke: from the center to the periphery [J]. Front Immunol, 2022, 13: 911661. DOI: 10.3389/fimmu.2022.911661. [9] O'Connell GC, Petrone AB, Treadway MB, et al. Machine-learning approach identifies a pattern of gene expression in peripheral blood that can accurately detect ischemic stroke [J]. NPJ Genom Med, 2016, 1: 16038. DOI: 10.1038/npjgenmed.2016.38. [10] Yang XT, Wang PY, Yan SQ, et al. Study on potential differentially expressed genes in stroke by bioinformatics analysis [J]. Neurol Sci, 2022, 43(2): 1155-1166. DOI: 10.1007/s10072-021-05470-1. [11] Stamova B, Jickling GC, Ander BP, et al. Gene expression in peripheral immune cells following cardioembolic stroke is sexually dimorphic [J]. PLoS One, 2014, 9(7): e102550. DOI: 10.1371/journal.pone.0102550. [12] Bhattacharya S, Andorf S, Gomes L, et al. ImmPort: disseminating data to the public for the future of immunology [J]. Immunol Res, 2014, 58(2-3): 234-239. DOI: 10.1007/s12026-014-8516-1. [13] Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies [J]. Nucleic Acids Res, 2015, 43(7): e47. DOI: 10.1093/nar/gkv007. [14] Chen L, Zhang YH, Wang SP, et al. Prediction and analysis of essential genes using the enrichments of gene ontology and KEGG pathways [J]. PLoS One, 2017, 12(9): e0184129. DOI: 10.1371/journal.pone.0184129. [15] Chin CH, Chen SH, Wu HH, et al. CytoHubba: identifying hub objects and sub-networks from complex interactome [J]. BMC Syst Biol, 2014, 8 (Suppl 4): S11. DOI: 10.1186/1752-0509-8-S4-S11. [16] Newman AM, Liu CL, Green MR, et al. Robust enumeration of cell subsets from tissue expression profiles [J]. Nat Methods, 2015, 12(5): 453-457. DOI: 10.1038/nmeth.3337. [17] Lorne E, Dupont H, Abraham E. Toll-like receptors 2 and 4: initiators of non-septic inflammation in critical care medicine? [J]. Intensive Care Med, 2010, 36(11): 1826-1835. DOI: 10.1007/s00134-010-1983-5. [18] Lissauer ME, Johnson SB, Bochicchio GV, et al. Differential expression of toll-like receptor genes: sepsis compared with sterile inflammation 1 day before sepsis diagnosis [J]. Shock, 2009, 31(3): 238-244. DOI: 10.1097/SHK.0b013e3181834991. [19] Tajalli-Nezhad S, Karimian M, Beyer C, et al. The regulatory role of Toll-like receptors after ischemic stroke: neurosteroids as TLR modulators with the focus on TLR2/4 [J]. Cell Mol Life Sci, 2019, 76(3): 523-537. DOI: 10.1007/s00018-018-2953-2. [20] Zhong CK, Yang JY, Xu T, et al. Serum matrix metalloproteinase-9 levels and prognosis of acute ischemic stroke [J]. Neurology, 2017, 22;89(8): 805-812. DOI: 10.1212/WNL.0000000000004257. [21] McColl BW, Rothwell NJ, Allan SM. Systemic inflammatory stimulus potentiates the acute phase and CXC chemokine responses to experimental stroke and exacerbates brain damage via interleukin-1- and neutrophil-dependent mechanisms [J]. J Neurosci, 2007, 27(16): 4403-4412. DOI: 10.1523/JNEUROSCI.5376-06.2007. [22] Kuol N, Stojanovska L, Nurgali K, et al. PD-1/PD-L1 in disease [J]. Immunotherapy, 2018, 10(2): 149-160. DOI: 10.2217/imt-2017-0120. [23] Gelderblom M, Weymar A, Bernreuther C, et al. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke [J]. Blood, 2012, 120(18): 3793-3802. DOI: 10.1182/blood-2012-02-412726. [24] Shichita T, Sugiyama Y, Ooboshi H, et al. Pivotal role of cerebral interleukin-17-producing gammadeltaT cells in the delayed phase of ischemic brain injury [J]. Nat Med, 2009, 15(8): 946-950. DOI: 10.1038/nm.1999. [25] Cai W, Liu SX, Hu MY, et al. Functional dynamics of neutrophils after ischemic stroke [J]. Transl Stroke Res, 2020, 11(1): 108-121. DOI: 10.1007/s12975-019-00694-y. [26] Wu ZQ, Lei K, Li HZ, et al. Transcriptome-based network analysis related to M2-like tumor-associated macrophage infiltration identified VARS1 as a potential target for improving melanoma immunotherapy efficacy [J]. J Transl Med, 2022, 20(1): 489. DOI: 10.1186/s12967-022-03686-z. -





 下载:
下载: