The mediation analysis of DNA methylation in the relationship between maternal arsenic exposure and neonatal birth weight
-
摘要:
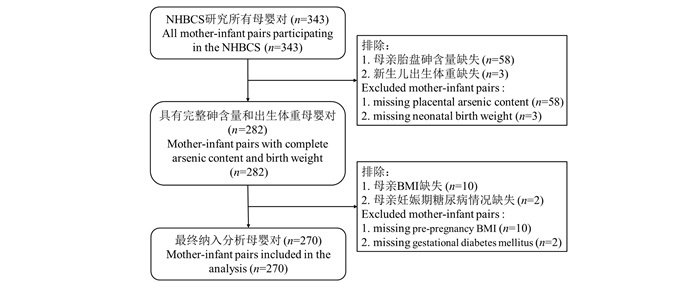
目的 探讨DNA甲基化(DNA methylation, DNAm)水平的改变是否在孕期砷暴露与新生儿出生体重之间有中介作用。 方法 资料来自新罕布什尔州出生队列研究,研究对象为2012年2月―2013年9月入选该队列的343对母婴,数据可从高通量基因表达(gene expression omnibus, GEO)数据库获取,检索号为GSE71678,共有270对母婴纳入本研究。采用确定独立筛选(sure independence screening, SIS)策略和中介效应分析方法估计DNAm位点在胎盘砷暴露与出生体重之间的中介效应。 结果 调整孕妇年龄、孕前BMI、妊娠期糖尿病、胎龄、新生儿性别、胎盘组织细胞成分后,筛选出25个候选中介DNAm位点。中介效应分析结果显示,通过Bonferroni法校正后,识别出位于VENTX基因上的cg14900295在孕期砷暴露致低出生体重有中间的作用(ACME=0.057 6, 95% CI:0.017 6~0.106 4, P=0.000 8);通过FDR方法校正,识别出18个胞嘧啶-磷酸-鸟嘌呤双核苷酸(cytosine-phosphate-guanine pairs of nucleotides, CpG)位点有中介作用,其中cg03348978、cg02435495、cg09463047、cg11862993均位于HNF1B基因。 结论 在妊娠期砷暴露导致低出生体重的过程中,共有18个特异性位点的DNAm水平改变起到了重要的中介作用,VENTX和HNF1B基因可能是其病因机制中的关键环节,为揭示砷暴露致低出生体重的遗传病因机制提供了参考依据。 Abstract:Objective To investigate whether the changes of DNA methylation level mediate the relationship between prenatal arsenic exposure and neonatal birth weight. Methods Data of this study were derived from the New Hampshire Birth Cohort Study, with 343 mother-infant pairs enrolled in the cohort from February 2012 to September 2013 and available from the GEO database under accession number GSE71678. A total of 270 maternal and infant pairs were included in this study. The Sure independence screening (SIS) strategy and mediation analysis method were employed to estimate the mediation effect of DNA methylation sites between placental arsenic exposure and birth weight. Results After adjusting for maternal age, pre-pregnancy BMI, gestational diabetes mellitus, gestational age, infant gender, and placental tissue cell composition, 25 DNA methylation sites were selected as candidate mediators. The results of mediation analysis showed that after corrected by Bonferroni method. cg14900295 located on the VENTX gene was identified as a mediator between maternal arsenic exposure and low birth weight (ACME=0.057 6, 95% CI: 0.017 6-0.106 4, P=0.000 8). After corrected by FDR method, 18 CpG loci were identified as mediators, among which cg03348978, cg02435495, cg09463047 and cg11862993 were all located on the HNF1B gene. Conclusions This study identified 18 specific sites where changes in DNA methylation levels play a crucial mediating role in the process linking prenatal arsenic exposure to low birth weight. Genes VENTX and HNF1B may be the key factors in the etiological mechanism. Therefore, these findings can provide a reference for revealing the genetic etiology mechanism of arsenic-induced low birth weight. -
Key words:
- DNA methylation /
- Placental arsenic exposure /
- Birth weight /
- Mediation effect
-
表 1 NHBCS研究对象基线特征
Table 1. Baseline characteristics of NHBCS subjects
变量 Variables NHBCS研究对象 NHBCS subjects① 母亲孕龄/岁 Maternal age/years 31.51(28.02, 34.93) 孕前母亲BMI
Pre-pregnancy maternal BMI/(kg·m-2)25.16(22.31, 29.10) 妊娠期糖尿病
Gestational diabetes mellitus是 Yes 28(10.37) 否 No 242(89.63) 胎龄/周 Gestational age / weeks 39.57(39.00, 40.43) 新生儿性别 Infant gender 男性 Male 139(51.48) 女性 Female 131(48.52) 新生儿出生体重 Infant birth weight/g 3440(3130, 3709) 胎盘砷含量 Placental arsenic levels/(ng·g-1) 0.8132(0.5468, 1.2277) 注:NHBCS,新罕布什尔州出生队列研究。
①以人数(占比/%)或M(P25, P75)表示。
Note: NHBCS, New Hampshire birth cohort study.
① Number of people (proportion/%) or M(P25, P75).表 2 25个候选CpG位点集的中介效应分析结果
Table 2. Mediation analysis results of 25 candidate CpG sites
CpGs CHR 基因 Gene Pα Pβ Pmax PME PME, Bon PME, FDR cg14900295 chr10 VENTX 0.000 9 0.000 3 0.000 9 0.000 8 0.020 0 ① 0.020 0 ① cg03348978 chr17 HNF1B 0.001 9 0.000 4 0.001 9 0.006 0 0.150 0 0.028 8 ① cg02435495 chr17 HNF1B 0.003 4 0.000 2 0.003 4 0.007 8 0.195 0 0.028 8 ① cg13814374 chr18 APCDD1 0.005 2 0.003 5 0.005 2 0.009 6 0.240 0 0.028 8 ① cg10205979 chr3 VPRBP 0.003 3 0.000 6 0.003 3 0.010 0 0.250 0 0.028 8 ① cg18676393 chr2 GEMIN6 0.002 4 0.001 5 0.002 4 0.010 8 0.270 0 0.028 8 ① cg18829186 chr14 TMED10 0.001 4 0.001 2 0.001 4 0.011 0 0.275 0 0.028 8 ① cg17200564 chr11 EIF3F 0.004 9 0.004 8 0.004 9 0.011 2 0.280 0 0.028 8 ① cg11939075 chr4 COPS4 0.000 4 0.000 9 0.000 9 0.012 0 0.300 0 0.028 8 ① cg02216727 chr17 GJD3 0.004 1 0.002 3 0.004 1 0.012 4 0.310 0 0.028 8 ① cg22863744 chr12 NCOR2 0.002 4 0.001 2 0.002 4 0.012 8 0.320 0 0.028 8 ① cg09463047 chr17 HNF1B 0.001 0 0.002 0 0.002 0 0.014 0 0.350 0 0.028 8 ① cg11862993 chr17 HNF1B 0.001 8 0.000 9 0.001 8 0.015 0 0.375 0 0.028 8 ① cg24138419 chr18 HDHD2 0.002 2 0.002 3 0.002 3 0.017 6 0.440 0 0.031 4 ① cg06400704 chr1 FMN2 0.001 9 0.004 7 0.004 7 0.024 2 0.605 0 0.040 3 ① cg04290595 — — 0.002 7 0.005 3 0.005 3 0.032 4 0.810 0 0.048 6 ① cg07249742 chr3 ITGB5 0.000 1 0.001 5 0.001 5 0.034 2 0.855 0 0.048 6 ① cg02908438 chr7 CPA1 0.002 3 0.001 1 0.002 3 0.035 0 0.875 0 0.048 6 ① cg00834223 chr2 AGAP1 0.003 8 0.000 6 0.003 8 0.056 4 1.000 0 0.074 2 cg17606548 chr5 MAST4 0.004 7 0.000 4 0.004 7 0.089 8 1.000 0 0.111 0 cg15827101 chr14 KLHDC2 0.002 8 0.005 0 0.005 0 0.093 2 1.000 0 0.111 0 cg20916535 chr6 VGLL2 0.000 1 0.004 8 0.004 8 0.111 2 1.000 0 0.126 4 cg27177983 chr4 LGI2 0.003 3 0.004 6 0.004 6 0.167 6 1.000 0 0.182 2 cg17156276 chr1 KDM4A 0.003 9 0.001 0 0.003 9 0.245 2 1.000 0 0.255 4 cg22763407 chr12 ELK3 0.005 1 0.004 9 0.005 1 0.299 2 1.000 0 0.299 2 注:CpG,胞嘧啶-磷酸-鸟嘌呤双核苷酸;Pα为中介模型中胎盘砷暴露的回归系数显著性;Pβ为结局模型中DNA甲基化位点的回归系数显著性;Pmax为Pα与Pβ二者的较大值;PME为因果中介效应的显著性;PME, Bon为对采用Bonferroni法校正P值;PME, FDR为对采用FDR法校正P值; “—”表示无法获取。
①表示经校正后P < 0.05。
Note: CpG, cytosine-phosphate-guanine pairs of nucleotides; Pα means the regression coefficient significance of placental arsenic exposure in the mediation model; Pβ means the regression coefficient significance of DNAm in the outcome model; Pmax means the maximum value of Pα and Pβ; PME means the significance of causal mediation effect; PME, Bon means the p values corrected by Bonferroni method; PME, FDR means the p values corrected by FDR method; "—" indicates that it cannot be obtained.
① Means the corrected P < 0.05.表 3 VENTX和HNF1B基因上5个CpG位点的中介分析结果
Table 3. Mediation analysis results of 5 CpG sites on the VENTX and HNF1B genes
CpGs 基因
GeneACME ADE TE 估计值Estimate
(95% CI)P值
value估计值Estimate
(95% CI)P值
value估计值Estimate
(95% CI)P值
valuecg14900295 VENTX 0.057 6(0.017 6, 0.106 4) 0.000 8 -0.091 3(-0.208 5, 0.066 7) 0.256 8 -0.033 7(-0.159 2, 0.119 4) 0.684 2 cg03348978 HNF1B 0.050 2(0.012 2, 0.088 3) 0.006 0 -0.076 7(-0.191 9, 0.080 4) 0.367 2 -0.026 5(-0.155 8, 0.123 5) 0.758 2 cg02435495 HNF1B 0.050 2(0.010 8, 0.092 8) 0.007 8 -0.077 1(-0.190 7, 0.079 5) 0.362 8 -0.026 9(-0.157 5, 0.123 1) 0.754 2 cg09463047 HNF1B 0.047 6(0.008 1, 0.090 9) 0.014 0 -0.072 4(-0.192 5, 0.088 5) 0.416 4 -0.024 8(-0.156 5, 0.125 8) 0.766 2 cg11862993 HNF1B 0.048 4(0.007 3, 0.089 5) 0.015 0 -0.071 6(-0.189 4, 0.089 4) 0.423 6 -0.023 1(-0.155 8, 0.125 6) 0.771 4 注:CpG,胞嘧啶-磷酸-鸟嘌呤双核苷酸;ACME,平均因果中介效应;ADE,平均直接效应;TE,总效应。
Note: CpG, cytosine-phosphate-guanine pairs of nucleotides; ACME, average causal mediation effect; ADE, average direct effect; TE, total effect. -
[1] Barker DJ. The fetal and infant origins of adult disease[J]. BMJ, 1990, 301(6761): 1111. DOI: 10.1136/bmj.301.6761.1111. [2] Wadhwa PD, Buss C, Entringer S, et al. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms[J]. Semin Reprod Med, 2009, 27(5): 358-368. DOI: 10.1055/s-0029-1237424. [3] Gluckman PD, Hanson MA, Cooper C, et al. Effect of in utero and early-life conditions on adult health and disease[J]. N Engl J Med, 2008, 359(1): 61-73. DOI: 10.1056/NEJMra0708473. [4] Jamnik T, Flasch M, Braun D, et al. Next-generation biomonitoring of the early-life chemical exposome in neonatal and infant development[J]. Nat Commun, 2022, 13(1): 2653. DOI: 10.1038/s41467-022-30204-y. [5] Shih YH, Scannell Bryan M, Argos M. Association between prenatal arsenic exposure, birth outcomes, and pregnancy complications: an observational study within the national children's study cohort[J]. Environ Res, 2020, 183: 109182. DOI: 10.1016/j.envres.2020.109182. [6] Green BB, Karagas MR, Punshon T, et al. Epigenome-wide assessment of DNA methylation in the placenta and arsenic exposure in the new Hampshire birth cohort study (USA)[J]. Environ Health Perspect, 2016, 124(8): 1253-1260. DOI: 10.1289/ehp.1510437. [7] Dye CK, Domingo-Relloso A, Kupsco A, et al. Maternal DNA methylation signatures of arsenic exposure is associated with adult offspring insulin resistance in the strong heart study[J]. Environ Int, 2023, 173: 107774. DOI: 10.1016/j.envint.2023.107774. [8] Küpers LK, Monnereau C, Sharp GC, et al. Meta-analysis of epigenome-wide association studies in neonates reveals widespread differential DNA methylation associated with birthweight[J]. Nat Commun, 2019, 10(1): 1893. DOI: 10.1038/s41467-019-09671-3. [9] Yuan V, Hui D, Yin YF, et al. Cell-specific characterization of the placental methylome[J]. BMC Genomics, 2021, 22(1): 6. DOI: 10.1186/s12864-020-07186-6. [10] Houseman EA, Accomando WP, Koestler DC, et al. DNA methylation arrays as surrogate measures of cell mixture distribution[J]. BMC Bioinformatics, 2012, 13: 86. DOI: 10.1186/1471-2105-13-86. [11] Fan JQ, Lyu JC. Sure independence screening for ultrahigh dimensional feature space[J]. J R Stat Soc Ser B Stat Methodol, 2008, 70(5): 849-911. DOI: 10.1111/j.1467-9868.2008.00674.x. [12] Tingley D, Yamamoto T, Hirose K, et al. Mediation: R package for causal mediation analysis[J]. J Stat Soft, 2014, 59(5): 1-38. DOI: 10.18637/jss.v059.i05. [13] Kumar S, Kumar V, Li WC, et al. Ventx family and its functional similarities with nanog: involvement in embryonic development and cancer progression[J]. Int J Mol Sci, 2022, 23(5): 2741. DOI: 10.3390/ijms23052741. [14] Wang GY, Yu J, Yang YW, et al. Whole-transcriptome sequencing uncovers core regulatory modules and gene signatures of human fetal growth restriction[J]. Clin Transl Med, 2020, 9(1): 9. DOI: 10.1186/s40169-020-0259-0. [15] Bozack AK, Rifas-Shiman SL, Coull BA, et al. Prenatal metal exposure, cord blood DNA methylation and persistence in childhood: an epigenome-wide association study of 12 metals[J]. Clin Epigenetics, 2021, 13(1): 208. DOI: 10.1186/s13148-021-01198-z. [16] Wang X, Wu H, Yu WH, et al. Hepatocyte nuclear factor 1b is a novel negative regulator of white adipocyte differentiation[J]. Cell Death Differ, 2017, 24(9): 1588-1597. DOI: 10.1038/cdd.2017.85. [17] Bozack AK, Domingo-Relloso A, Haack K, et al. Locus-specific differential DNA methylation and urinary arsenic: an epigenome-wide association study in blood among adults with low-to-moderate arsenic exposure[J]. Environ Health Perspect, 2020, 128(6): 67015. DOI: 10.1289/EHP6263. -





 下载:
下载: